Deck 2: Atoms and Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 2: Atoms and Elements
1
Which statement is incorrect?
A) According to the atomic theory, all matter is composed of atoms.
B) Protons, neutrons, and electrons are subatomic particles.
C) Electrons have greater mass than protons.
D) Neutrons are found in the nucleus of the atom.
A) According to the atomic theory, all matter is composed of atoms.
B) Protons, neutrons, and electrons are subatomic particles.
C) Electrons have greater mass than protons.
D) Neutrons are found in the nucleus of the atom.
Electrons have greater mass than protons.
2
Choose the incorrect statement about the proton:
A) The proton has the atomic mass of 1 amu.
B) The proton has the same charge as the neutron.
C) The proton has greater mass than an electron.
D) The proton and the neutron have approximately the same atomic mass.
A) The proton has the atomic mass of 1 amu.
B) The proton has the same charge as the neutron.
C) The proton has greater mass than an electron.
D) The proton and the neutron have approximately the same atomic mass.
The proton has the same charge as the neutron.
3
The atom's structure characteristically has
A) the protons and neutron within the nucleus.
B) the electrons located outside the nucleus.
C) mostly empty space.
D) All of these choices are correct.
A) the protons and neutron within the nucleus.
B) the electrons located outside the nucleus.
C) mostly empty space.
D) All of these choices are correct.
All of these choices are correct.
4
Which of the following is not a type of subatomic particle?
A) alpha particle
B) electron
C) neutron
D) proton
A) alpha particle
B) electron
C) neutron
D) proton

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the substances listed below is not an element?
A) sodium
B) iron
C) air
D) carbon
A) sodium
B) iron
C) air
D) carbon

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
A Nutrition Facts label on a food package indicates that one serving contains 2% of the recommended daily allowance (RDA) of iodine. If the serving size is one cup and the RDA for iodine is 0.15 mg per day, calculate the amount (in milligrams) supplied by one serving.
A) 0.0030 mg
B) 15 mg
C) 20 mg
D) 0.15 mg
A) 0.0030 mg
B) 15 mg
C) 20 mg
D) 0.15 mg

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
The mass in grams of one mole of an element is the element's ___.
A) atomic number
B) atomic weight
C) molar mass
D) isotope number
A) atomic number
B) atomic weight
C) molar mass
D) isotope number

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
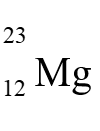 has how many protons, neutrons, and electrons?
has how many protons, neutrons, and electrons?A) 12 protons, 23 neutrons, and 23 electrons
B) 12 protons, 11 neutrons, and 11 electrons
C) 23 protons, 11 neutrons, and 23 electrons
D) 12 protons, 11 neutrons, and 12 electrons

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
Which is the proper notation for the oxygen isotope with atomic number 8 and a mass number of 16?
A)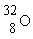
B)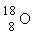
C)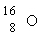
D)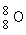
A)
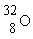
B)
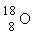
C)
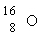
D)
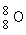

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
An isotope of lithium contains 3 protons and 4 neutrons. What is the correct notation for this lithium isotope?
A)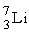
B)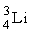
C)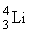
D)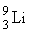
A)
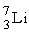
B)
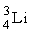
C)
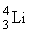
D)
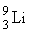

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
Two atoms that are isotopes of an element contain
A) the same number of electrons and neutrons.
B) the same number of protons and neutrons.
C) the same number of protons and a different number of neutrons.
D) different numbers of protons.
A) the same number of electrons and neutrons.
B) the same number of protons and neutrons.
C) the same number of protons and a different number of neutrons.
D) different numbers of protons.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an isotope of iodine-131?
A)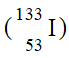
B)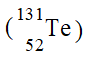
C)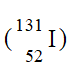
D)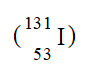
A)
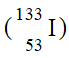
B)
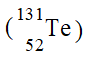
C)
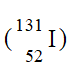
D)
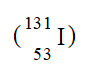

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
Atoms of elements belonging to the same group have an identical number of
A) total electrons.
B) energy levels.
C) inner electrons.
D) valence electrons.
A) total electrons.
B) energy levels.
C) inner electrons.
D) valence electrons.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement is correct?
A) Cr is in period 6B.
B) Fe is a transition metal.
C) Mg is an alkali metal.
D) Cl is an inert gas.
A) Cr is in period 6B.
B) Fe is a transition metal.
C) Mg is an alkali metal.
D) Cl is an inert gas.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the correct order of elements in increasing atomic radius: K, Mg, B?
A) KB) BC) MgD) B
A) K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
The periodic table of the elements does not list whole numbers for atomic weights. Why?
A) The atomic weights are not predictable.
B) The atomic weights include protons and neutrons at 1 amu each, but they also include electrons, which weigh a lot less than one.
C) The atomic weight is the weighted average of the masses of the known isotopes of an element.
D) The atomic weights do not include isotopes.
A) The atomic weights are not predictable.
B) The atomic weights include protons and neutrons at 1 amu each, but they also include electrons, which weigh a lot less than one.
C) The atomic weight is the weighted average of the masses of the known isotopes of an element.
D) The atomic weights do not include isotopes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
Where are the metals located on the periodic table of the elements?
A) On the right side of the metalloid line (not including the metalloids).
B) On the last group of the periodic table.
C) On the left side of the metalloid line (not including the methalloids).
D) In the two sections separated from the rest of the table.
A) On the right side of the metalloid line (not including the metalloids).
B) On the last group of the periodic table.
C) On the left side of the metalloid line (not including the methalloids).
D) In the two sections separated from the rest of the table.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
The higher up within a group or family on the periodic table,
A) the heavier the nucleus.
B) the more the element is likely to conduct electricity.
C) the more nonmetallic in character.
D) the more the element is likely to be a semimetal.
A) the heavier the nucleus.
B) the more the element is likely to conduct electricity.
C) the more nonmetallic in character.
D) the more the element is likely to be a semimetal.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
Boron, which occurs in nature as 105B (amu = 10.013) and 115B (amu = 11.009), has an atomic weight of 10.81. Which of the following statements is true?
A) isotope115B predominates
B) isotope105B predominates
C) both isotopes have equal percentages in nature
D) the atomic weight of 10.013 amu is nearest the correct value for boron
A) isotope115B predominates
B) isotope105B predominates
C) both isotopes have equal percentages in nature
D) the atomic weight of 10.013 amu is nearest the correct value for boron

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
Glucose molecules produced during photosynthesis contain higher levels of carbon-12 than carbon -13 because
A) carbon -12 reacts slightly slower than carbon-13.
B) carbon-12 has more electrons than carbon -13.
C) carbon-12 reacts slightly faster than carbon-13.
D) carbon-13 has more number of protons than carbon -12.
A) carbon -12 reacts slightly slower than carbon-13.
B) carbon-12 has more electrons than carbon -13.
C) carbon-12 reacts slightly faster than carbon-13.
D) carbon-13 has more number of protons than carbon -12.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
What is the relationship of a metal atom to the right of another metal atom in the same period?
A) The atom to the right is usually the smaller in diameter.
B) The atom to the right is usually the smaller in atomic weight.
C) The atom to the right is usually the smaller in atomic number.
D) The atom to the right is more likely to have fewer isotopes.
A) The atom to the right is usually the smaller in diameter.
B) The atom to the right is usually the smaller in atomic weight.
C) The atom to the right is usually the smaller in atomic number.
D) The atom to the right is more likely to have fewer isotopes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
How many atoms of sodium are there in 0.200 moles sodium atoms?
A) 6.02 x 1024 atoms
B) 6.02 x 1023 atoms
C) 1.20 x 1023 atoms
D) 1.20 x 1024atoms
A) 6.02 x 1024 atoms
B) 6.02 x 1023 atoms
C) 1.20 x 1023 atoms
D) 1.20 x 1024atoms

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
How many carbon(C) atoms are present in 30 moles of carbon?
A) 1.20 x 101C atoms
B) 1.80 x 1023 C atoms
C) 3.60 x 102 C atoms
D) 6.02 x 1023 C atoms
A) 1.20 x 101C atoms
B) 1.80 x 1023 C atoms
C) 3.60 x 102 C atoms
D) 6.02 x 1023 C atoms

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
The number of moles of calcium (Ca) represented by 7.39 g of calcium is
A) 40.0 moles of Ca.
B) 18.6 moles of Ca.
C) 0.184 mole of Ca.
D) 296 moles of Ca.
A) 40.0 moles of Ca.
B) 18.6 moles of Ca.
C) 0.184 mole of Ca.
D) 296 moles of Ca.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
Iron has the atomic weight of 55.9. What is the number of atoms in one mole of iron?
A) 55.9 atoms
B) 1 x 1024 atoms
C) 6.02 x 1023 atoms
D) 12 dozen atoms
A) 55.9 atoms
B) 1 x 1024 atoms
C) 6.02 x 1023 atoms
D) 12 dozen atoms

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
A sample of magnesium weighs 10 grams. How many moles of magnesium are there in the sample?
A) 10 moles
B) 6 x 1023 moles
C) 60 x 1023 atoms
D) 0.4 moles
A) 10 moles
B) 6 x 1023 moles
C) 60 x 1023 atoms
D) 0.4 moles

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
What is the number of atoms present in a 20.gram sample of lithium?
A) 1.38 x 102
B) 1.20 x 1025
C) 1.70 x 1024
D) 4.00x 1024
A) 1.38 x 102
B) 1.20 x 1025
C) 1.70 x 1024
D) 4.00x 1024

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
Sulfur has ___ electrons and ___ valence electrons.
A) 16, 6
B) 32, 4
C) 16, 0
D) 32, 6
A) 16, 6
B) 32, 4
C) 16, 0
D) 32, 6

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
An alpha particle is identical to which of the following?
A) the nucleus of a hydrogen atom
B) an electron
C) a beta radiation
D) the nucleus of a helium-4-atom
A) the nucleus of a hydrogen atom
B) an electron
C) a beta radiation
D) the nucleus of a helium-4-atom

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
In an alpha therapy, Radium-223is used as a radioactive -emitter. The correct nuclear equation for this emission event is?
A)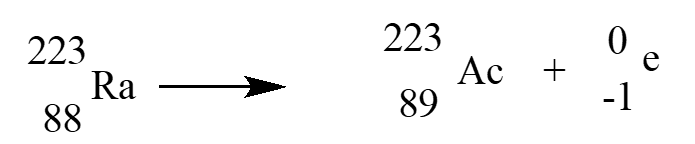
B)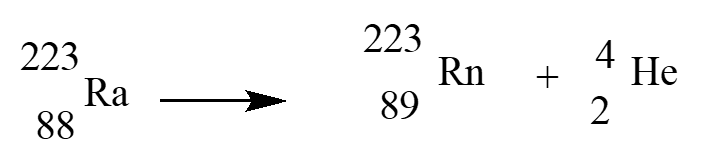
C)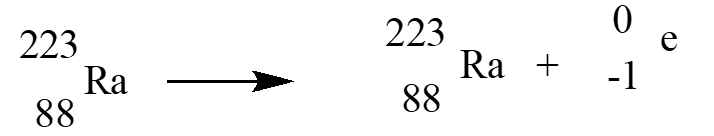
D)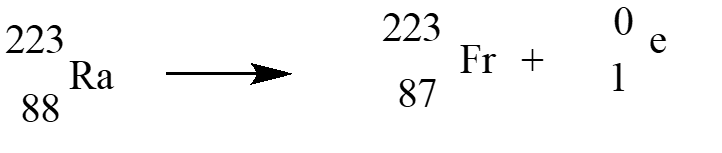
A)
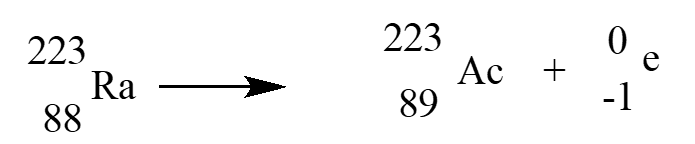
B)
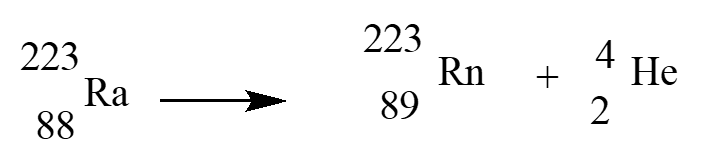
C)
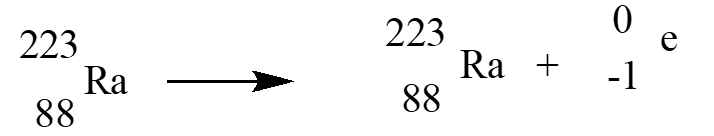
D)
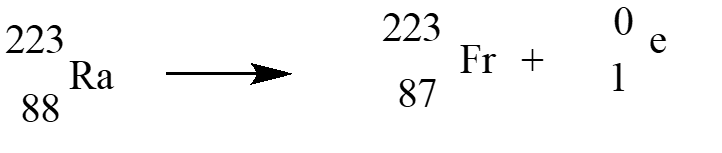

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is true about a beta particle?
A) it has the same mass as a helium nucleus
B) when ejected from the nucleus formed has same mass as the original isotope
C) when ejected from the nucleus of a radioisotope, the nucleus formed has one more proton
D) it is the same as a gamma radiation
A) it has the same mass as a helium nucleus
B) when ejected from the nucleus formed has same mass as the original isotope
C) when ejected from the nucleus of a radioisotope, the nucleus formed has one more proton
D) it is the same as a gamma radiation

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
When a radioactive isotope emits a(n)___, its atomic number increases.
A) alpha particle
B) beta particle
C) gamma ray
D) positron
A) alpha particle
B) beta particle
C) gamma ray
D) positron

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
If radium, 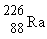 , were to emit an alpha particle, what would be the other product?
, were to emit an alpha particle, what would be the other product?
A)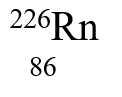
B)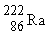
C)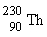
D)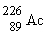
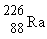 , were to emit an alpha particle, what would be the other product?
, were to emit an alpha particle, what would be the other product?A)
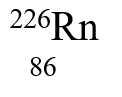
B)
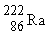
C)
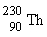
D)
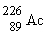

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
Scandium-40 enters into a reaction in which a positron is emitted. Which is the correct equation for this reaction?
A)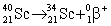
B)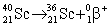
C)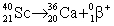
D)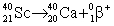
A)
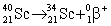
B)
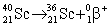
C)
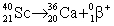
D)
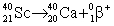

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
Which equation below correctly depicts nitrogen-13 emitting a positron?
A)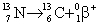
B)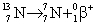
C)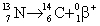
D)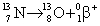
A)
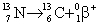
B)
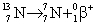
C)
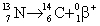
D)
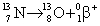

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
The dosage unit related to the amount of energy absorbed by an object exposed to nuclear radiation is ___.
A) curie
B) rad
C) rem
D) Becquerel
A) curie
B) rad
C) rem
D) Becquerel

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
Radioisotopes with short half-lives are used in diagnostic tests because
A) they have high energy.
B) they are inexpensive.
C) they do not pose health risks by being present long after use.
D) they have less penetration ability.
A) they have high energy.
B) they are inexpensive.
C) they do not pose health risks by being present long after use.
D) they have less penetration ability.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Most elements have radioisotopes. What is a radioisotope?
A) An isotope that has a different atomic number.
B) An isotope that releases nuclear radiation.
C) An isotope that releases electrical radiation.
D) Any isotope that can absorb nuclear particles.
A) An isotope that has a different atomic number.
B) An isotope that releases nuclear radiation.
C) An isotope that releases electrical radiation.
D) Any isotope that can absorb nuclear particles.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
What is the minimum protection against exposure to beta particles?
A) None, beta particles are of low energy and cannot penetrate skin.
B) Gloves, a lab coat, or over a meter's distance from the source.
C) A thin sheet of plastic or metal.
D) A sheet of lead.
A) None, beta particles are of low energy and cannot penetrate skin.
B) Gloves, a lab coat, or over a meter's distance from the source.
C) A thin sheet of plastic or metal.
D) A sheet of lead.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
Gamma rays require a thick slab of concrete or lead to block them because they
A) have the lowest energy.
B) are so energetic that they have a very high penetrating power.
C) are large in size.
D) are small in size.
A) have the lowest energy.
B) are so energetic that they have a very high penetrating power.
C) are large in size.
D) are small in size.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
The quality factor (QF) for alpha particles is 20. What is the radiation dose in remsfor this patient if he absorbed 13 rads of alpha particles?
A) 20 rems
B) 13 rems
C) 23 rems
D) 260 rems
A) 20 rems
B) 13 rems
C) 23 rems
D) 260 rems

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
If one starts with 10.0 grams of a radioactive substance, how much will remain after 3 half lives?
A) 1.25 g
B) 2.50 g
C) 3.33 g
D) 5.00 g
A) 1.25 g
B) 2.50 g
C) 3.33 g
D) 5.00 g

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
Iron-59 has a half-life of 45 days. How much of a 50.0-gram sample would remain after 180 days?
A) 1.00 g
B) 3.13 g
C) 25.0 g
D) There is no way to determine the results.
A) 1.00 g
B) 3.13 g
C) 25.0 g
D) There is no way to determine the results.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
A 35 mg dose of iodine-131 with a half-life of 8 days is administered to a patient. How long will it take for the iodine to decay so that there are only about 2 mg remaining?
A) 8 days
B) 16 days
C) 32 days
D) 64 days
A) 8 days
B) 16 days
C) 32 days
D) 64 days

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
Whereas x-ray works well for bones, the value of a CT scan is that
A) it provides a 3 dimensional view of bones.
B) it provides images of the inside of the body.
C) it provides a clear picture of gamma rays produced within the tissue.
D) it provides images used in studies, such as monitoring sugar usage.
A) it provides a 3 dimensional view of bones.
B) it provides images of the inside of the body.
C) it provides a clear picture of gamma rays produced within the tissue.
D) it provides images used in studies, such as monitoring sugar usage.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Whereas x-ray works well for bones, the value of MRI imaging is that
A) it provides a 3 dimensional view of bones.
B) it provides images of the inside of the body.
C) it provides a clear picture of gamma rays produced within the tissue.
D) it provides images used in studies, such as monitoring sugar usage.
A) it provides a 3 dimensional view of bones.
B) it provides images of the inside of the body.
C) it provides a clear picture of gamma rays produced within the tissue.
D) it provides images used in studies, such as monitoring sugar usage.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
A patient who has undergone a PET scan that used fluorine-18 tagged glucose is likely to emit which of the following a few hours after the procedure?
A) x-rays
B) alpha particles
C) gamma radiation
D) beta particles
A) x-rays
B) alpha particles
C) gamma radiation
D) beta particles

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
Trace elements and vitamins are required for the body to function properly

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
An atom contains 12 protons and 12 neutrons, and a different atom contains 10 protons and 12 neutrons. These two atoms are not isotopes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
The isotope of hydrogen that is a radioisotope has a mass number of 3.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
Calcium is a transition metal as is indicated by its location in the periodic table.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
Emission of a beta particle increases the number of protons in an atom's nucleus.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
The emission of an alpha particle only decreases the atomic mass of the atom.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
The higher the quality factor (QF) of a radiation, the less harmful the exposure to the radiation.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
The element that is essential for human nutrition and is responsible for formation of bones and teeth is ___.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
The atomic number is equal to the number of ___ found in a specific element.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
Complete the following table:
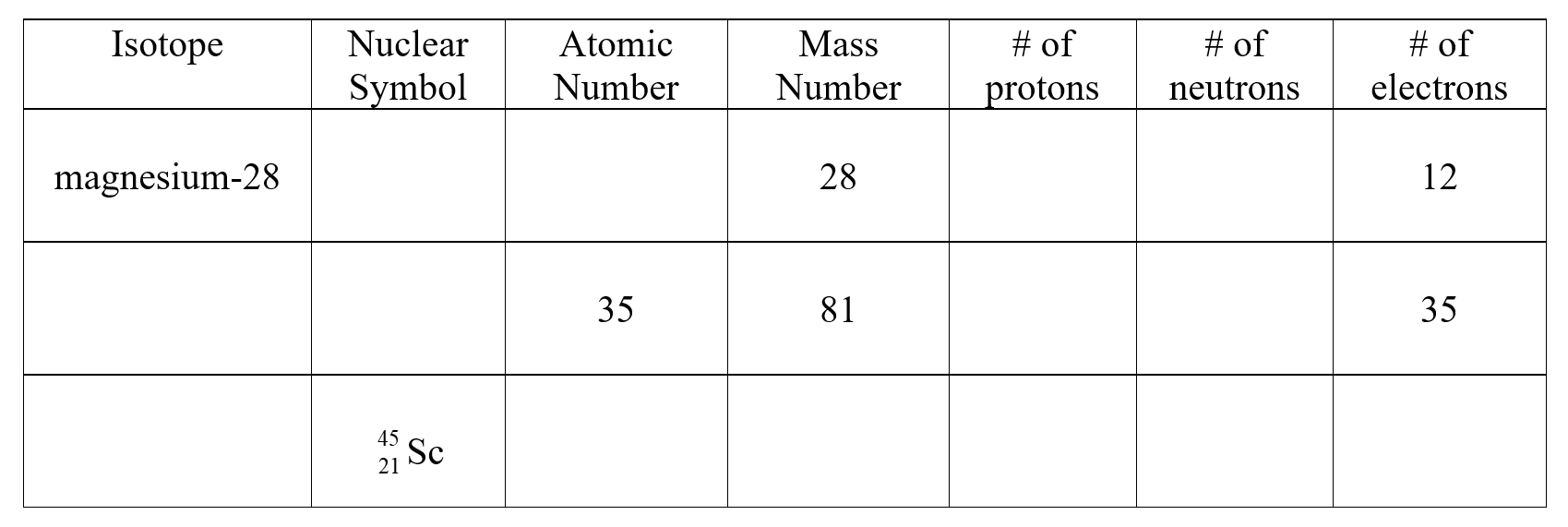


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
Complete the following table:
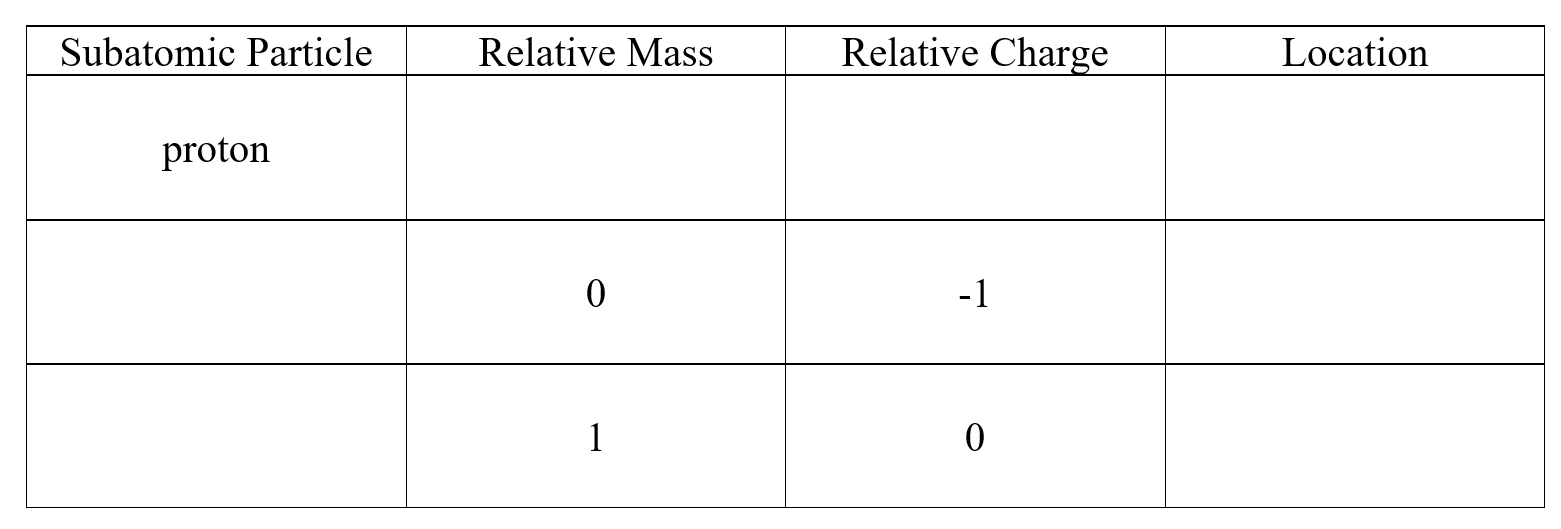


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Beryllium and magnesium can be predicted to have similar properties because they are in the same ___ on the periodic table.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
Given the following elements: calcium, selenium, and chlorine,arrange the three elements in order of:
increasing atomic size: _____<_____<_____
increasing metallic character _____>_____>_____
increasing atomic size: _____<_____<_____
increasing metallic character _____>_____>_____

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
The number of ___ is the same in one mole of sodium as in one mole of zinc.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
In a living thing, light can be produced without heat in a process called ___.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
An alpha particle is essentially a(n) ___ nucleus.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
___ radiation is electromagnetic radiation much like x-rays.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
What type of radiation will be emitted in the reaction below?
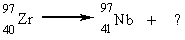
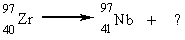

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
Complete the equation below.
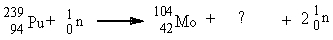
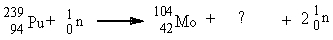

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
The least penetrating form of ionizing radiation is the ___ particle.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
The time required for one half of a sample to undergo radioactive decay is the ___.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
0.04 mSv is the same as ___millirads.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the number of moles in 2.61 grams of silicon.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
Calculate the number of atoms in 2.61 grams of silicon.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
Define the term mole. Explain the relationship between mole and atomic weight.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
What is the mass in grams of 5.82 x 1015 moles of Ba?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
12.5 grams of gold contains how many gold atoms?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
Draw the electron dot structure for a selenium (Se) atom.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
Write the nuclear equation for the alpha emission of bismuth-210.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
Technetium-99 is injected into a patient in order to monitor heart function. Technetium-99 is a gamma emitter with a half-life of 6 hours. A patient receives an injection of 21 mg of Technetium-99 on a Tuesday at 9:00 a.m.
Calculate the number of hours and the number of half-lives that occur between 9:00 a.m. on Tuesday and 11:00 p.m. on Wednesday.
Calculate the number of hours and the number of half-lives that occur between 9:00 a.m. on Tuesday and 11:00 p.m. on Wednesday.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
Define the term element. Give one example of an element that is a metal, an element that is a nonmetal, and an element that is a semimetal.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
Describe two ways to protect oneself from exposure to radiation when working in an environment that contains radioactive sources.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


