Deck 12: Intermolecular Attractions and the Properties of Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/189
Play
Full screen (f)
Deck 12: Intermolecular Attractions and the Properties of Liquids and Solids
1
The term "London forces"is a synonym for
A)ion-ion forces.
B)permanent dipole-permanent dipole interactions.
C)hydrogen bonding.
D)instantaneous dipole-instantaneous dipole interactions.
E)instantaneous dipole-induced dipole interactions.
A)ion-ion forces.
B)permanent dipole-permanent dipole interactions.
C)hydrogen bonding.
D)instantaneous dipole-instantaneous dipole interactions.
E)instantaneous dipole-induced dipole interactions.
instantaneous dipole-induced dipole interactions.
2
Which molecule is most polarizable and subject to significant instantaneous dipole-induced dipole forces?
A)H3C-Br
B)H3C-Cl
C)H3C-F
D)H3C-H
E)H3C-I
A)H3C-Br
B)H3C-Cl
C)H3C-F
D)H3C-H
E)H3C-I
H3C-I
3
Which molecule is most polarizable and subject to the greatest instantaneous dipole-induced dipole forces?
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12
C5H12
4
For small molecules of comparable molecular weight, which one of the following choices lists the intermolecular forces in the order of increasing strength?
A)hydrogen bonds < dipole-dipole forces < London forces
B)dipole-dipole forces < hydrogen bonds < London forces
C)London forces < hydrogen bonds < dipole-dipole forces
D)hydrogen bonds < London forces < dipole-dipole forces
E)London forces < dipole-dipole forces < hydrogen bonds
A)hydrogen bonds < dipole-dipole forces < London forces
B)dipole-dipole forces < hydrogen bonds < London forces
C)London forces < hydrogen bonds < dipole-dipole forces
D)hydrogen bonds < London forces < dipole-dipole forces
E)London forces < dipole-dipole forces < hydrogen bonds

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
5
What compound will not exhibit hydrogen bonding in the liquid state?
A)CH3-CH2-Br
B)CH3-CH2-NH2
C)CH3-CH2-CH2-OH
D)CH3-NH-CH3
E)NH2-OH
A)CH3-CH2-Br
B)CH3-CH2-NH2
C)CH3-CH2-CH2-OH
D)CH3-NH-CH3
E)NH2-OH

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
6
Which covalent compound will exhibit hydrogen bonding in the liquid state?
A)CH2F2
B)Cl2NH
C)H2PCl
D)HBr
E)NCl3
A)CH2F2
B)Cl2NH
C)H2PCl
D)HBr
E)NCl3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
7
Which covalent compound will exhibit hydrogen bonding in the liquid state?
A)CCl2F2
B)H2PCl
C)HCl
D)NH2OH
E)NF3
A)CCl2F2
B)H2PCl
C)HCl
D)NH2OH
E)NF3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
8
Which covalent compound will exhibit hydrogen bonding in the liquid state?
A)PH3
B)H2
C)CH4
D)NH3
E)H2S
A)PH3
B)H2
C)CH4
D)NH3
E)H2S

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
9
Which compound will have the strongest intermolecular forces?
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound will have the weakest intermolecular forces?
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
11
The strongest intermolecular forces between molecules of NO are
A)covalent bonds.
B)dipole-dipole interactions.
C)ionic bonds.
D)hydrogen bonds.
E)London forces.
A)covalent bonds.
B)dipole-dipole interactions.
C)ionic bonds.
D)hydrogen bonds.
E)London forces.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound is expected to exhibit hydrogen bonding?
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H
A)CH3-CH2-H
B)CH3-CH2-O-H
C)CH3-CH2-PH2
D)CH3-CH2-S-H
E)CH3-CH2-Se-H

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
13
At 1.0 atm pressure, ice (solid H2O)floats in water instead of sinking. The reason for this is
A)when water freezes, it expands instead of contracting.
B)the fusion process is endothermic, therefore the solid will float.
C)the triple point has a lower temperature than the freezing point for water.
D)the critical temperature has a higher temperature than the normal boiling point.
E)the triple point corresponds to a pressure below 1 standard atmosphere.
A)when water freezes, it expands instead of contracting.
B)the fusion process is endothermic, therefore the solid will float.
C)the triple point has a lower temperature than the freezing point for water.
D)the critical temperature has a higher temperature than the normal boiling point.
E)the triple point corresponds to a pressure below 1 standard atmosphere.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
14
The property that measures or describes the magnitude of resistance to flow in a liquid is called
A)London forces.
B)malleability.
C)surface tension.
D)vapor pressure.
E)viscosity.
A)London forces.
B)malleability.
C)surface tension.
D)vapor pressure.
E)viscosity.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
15
Which causes surface tension?
A)more molecules present on a surface than within a liquid
B)a liquid forming as much surface area as possible
C)surface molecules having fewer molecular neighbors than interior molecules
D)a higher viscosity within a liquid than at the surface of a liquid
E)the largest average size molecules moving toward the surface of a liquid
A)more molecules present on a surface than within a liquid
B)a liquid forming as much surface area as possible
C)surface molecules having fewer molecular neighbors than interior molecules
D)a higher viscosity within a liquid than at the surface of a liquid
E)the largest average size molecules moving toward the surface of a liquid

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
16
The presence of which intermolecular force would lead to the highest viscosity within a liquid?
A)dipole-dipole
B)London
C)dispersion
D)Hydrogen bonding
E)induced dipole
A)dipole-dipole
B)London
C)dispersion
D)Hydrogen bonding
E)induced dipole

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
17
When a liquid undergoes a change of state to a gas, the process is called
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
18
When a solid undergoes a change of state to a gas, the process is called
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
19
When a gas undergoes a change of state to a liquid, the process is called
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.
A)condensation.
B)deposition.
C)fusion.
D)sublimation.
E)evaporation.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is not a factor that directly affects the viscosity of a liquid?
A)length of the molecule
B)polarizability
C)density
D)branching of molecule
E)temperature
A)length of the molecule
B)polarizability
C)density
D)branching of molecule
E)temperature

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following organic liquids would best demonstrate wetting on a clean glass surface?
A)carbon tetrachloride (CCl4)
B)octane (C8H18)
C)hexane (C6H14)
D)ethylene glycol (HO-CH2-CH2-OH)
E)heptane (C7H16)
A)carbon tetrachloride (CCl4)
B)octane (C8H18)
C)hexane (C6H14)
D)ethylene glycol (HO-CH2-CH2-OH)
E)heptane (C7H16)

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following organic liquids would have the strongest surface tension?
A)ethanol (CH3-CH2-OH)
B)octane (C8H18)
C)hexane (C6H14)
D)ethylene glycol (HO-CH2-CH2-OH)
E)heptanes (C7H16)
A)ethanol (CH3-CH2-OH)
B)octane (C8H18)
C)hexane (C6H14)
D)ethylene glycol (HO-CH2-CH2-OH)
E)heptanes (C7H16)

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
23
A liquid in a covered container is in equilibrium with its vapor. If the cover is removed, what is the immediate result?
A)The average kinetic energy increases.
B)The evaporation rate decreases.
C)The evaporation rate increases.
D)The condensation rate decreases.
E)The condensation rate increases.
A)The average kinetic energy increases.
B)The evaporation rate decreases.
C)The evaporation rate increases.
D)The condensation rate decreases.
E)The condensation rate increases.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following liquids, at the same temperature, has the lowest vapor pressure?
A)CH3-CH2-F
B)CH3-CH2-CH3
C)CH3-CH2-CH2-CH3
D)CH3-CH2-O-H
E)CH3-O-CH3
A)CH3-CH2-F
B)CH3-CH2-CH3
C)CH3-CH2-CH2-CH3
D)CH3-CH2-O-H
E)CH3-O-CH3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following liquids, at the same temperature, has the lowest vapor pressure?
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following liquids, at the same temperature, has the highest vapor pressure?
A)CH3-CH2-H
B)CH3-O-CH3
C)CH3-CH2-CH2-CH3
D)CH3-CH2-CH3
E)CH3-CH2-S-H
A)CH3-CH2-H
B)CH3-O-CH3
C)CH3-CH2-CH2-CH3
D)CH3-CH2-CH3
E)CH3-CH2-S-H

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following liquids, at the same temperature, has the highest vapor pressure?
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following liquids, at the same temperature, has the highest vapor pressure?
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
29
The vapor pressure of a liquid increases with increasing temperature. The temperature at which the vapor pressure is equal to the prevailing outside atmospheric pressure is
A)the boiling point.
B)the flash point.
C)the vaporization point.
D)100 °C.
E)the normal boiling point.
A)the boiling point.
B)the flash point.
C)the vaporization point.
D)100 °C.
E)the normal boiling point.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
30
The temperature at which the vapor pressure is equal to 1 atm, 760 torr, or 101,325 Pa is defined as
A)the melting point.
B)the flash point.
C)the vaporization point.
D)100°C.
E)the normal boiling point.
A)the melting point.
B)the flash point.
C)the vaporization point.
D)100°C.
E)the normal boiling point.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound should have the lowest boiling point?
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound should have the lowest boiling point?
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
33
Which compound should have the highest boiling point?
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
34
Which compound should have the highest boiling point?
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3
A)CH3-Hg-CH3
B)CH3-O-CH3
C)CH3-S-CH3
D)CH3-Se-CH3
E)CH3-Te-CH3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
35
Which factor affects the normal boiling point of a liquid?
A)atmospheric pressure
B)rate of condensation
C)rate of evaporation
D)strength of the intermolecular forces
E)rate of temperature increase
A)atmospheric pressure
B)rate of condensation
C)rate of evaporation
D)strength of the intermolecular forces
E)rate of temperature increase

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following would be expected to have the lowest vapor pressure at room temperature?
A)dimethyl sulfoxide, boiling point = 189°C
B)1-pentanol, boiling point = 137°C
C)diethyl ether, boiling point = 34.6°C
D)acetone, boiling point = 56°C
E)The vapor pressure of each of the liquids at room temperature would be the same.
A)dimethyl sulfoxide, boiling point = 189°C
B)1-pentanol, boiling point = 137°C
C)diethyl ether, boiling point = 34.6°C
D)acetone, boiling point = 56°C
E)The vapor pressure of each of the liquids at room temperature would be the same.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
37
Given the following substances and their normal boiling points, in °C: 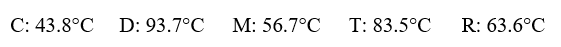 Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?
A)C > R > D
B)D > T > R
C)R > M > D
D)C > D > M
E)D > R > M
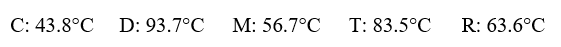 Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of decreasing vapor pressure at 20°C?A)C > R > D
B)D > T > R
C)R > M > D
D)C > D > M
E)D > R > M

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
38
Given the following substances and their normal boiling points, in °C:  Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?
A)C < R < D
B)D < R < C
C)R < T < D
D)C < D < M
E)D < M < R
 Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?A)C < R < D
B)D < R < C
C)R < T < D
D)C < D < M
E)D < M < R

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
39
Given the following substances and their normal boiling points, in °C: 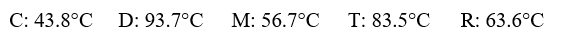 Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?
Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?
A)C > R > D
B)D > C > R
C)R > T > D
D)C > D > M
E)D > R > C
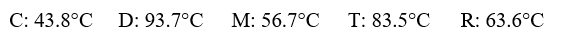 Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?
Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?A)C > R > D
B)D > C > R
C)R > T > D
D)C > D > M
E)D > R > C

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
40
Given the following substances and their normal boiling points, in °C:  Which ranking correctly lists some of these liquids in order of increasing intermolecular forces at 20°C?
Which ranking correctly lists some of these liquids in order of increasing intermolecular forces at 20°C?
A)C < R < D
B)D < T < R
C)R < T < C
D)C < D < M
E)D < R < M
 Which ranking correctly lists some of these liquids in order of increasing intermolecular forces at 20°C?
Which ranking correctly lists some of these liquids in order of increasing intermolecular forces at 20°C?A)C < R < D
B)D < T < R
C)R < T < C
D)C < D < M
E)D < R < M

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
41
In order to boil water, we need to supply heat. This is in order to
A)decrease the kinetic energy of the molecules.
B)disrupt ionic interactions.
C)disrupt hydrogen bonding.
D)decrease the potential energy of the molecules.
E)All of the above
A)decrease the kinetic energy of the molecules.
B)disrupt ionic interactions.
C)disrupt hydrogen bonding.
D)decrease the potential energy of the molecules.
E)All of the above

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
42
Benzene, C6H8, has an enthalpy of fusion of 127.4 J g-1 and its melting point temperature is 5.53°C. How much heat is required to transform 80.0 g of solid benzene at 5.53°C into liquid benzene, also at 5.53°C?
A)705 J
B)10.2 kJ
C)14.4 kJ
D)19.3 kJ
E)22.3 kJ
A)705 J
B)10.2 kJ
C)14.4 kJ
D)19.3 kJ
E)22.3 kJ

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
43
p-Xylene, C8H10, has an enthalpy of fusion of 158.3 J g-1 and its melting point temperature is 13.2°C. How much heat is required to transform 115 g of solid p-xylene at 13.2°C into liquid p-xylene, also at 13.2°C?
A)1.52 kJ
B)2.09 kJ
C)18.2 kJ
D)32.9 kJ
E)41.8 kJ
A)1.52 kJ
B)2.09 kJ
C)18.2 kJ
D)32.9 kJ
E)41.8 kJ

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
44
Benzophenone, C13H10O, has a heat of fusion of 98.45 J g-1 and its melting point temperature is 47.85°C. How much heat is required to transform 126 g of solid benzophenone at 47.85°C into liquid benzophenone, also at 47.85°C?
A)4.71 kJ
B)6.03 kJ
C)12.4 kJ
D)31.6 kJ
E)40.4 kJ
A)4.71 kJ
B)6.03 kJ
C)12.4 kJ
D)31.6 kJ
E)40.4 kJ

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
45
Substance A has a normal melting point of -25.0°C, a heat of fusion of 1200 J g-1; specific heats for the solid and the liquid are 3.00 and 6.20 J g-1 °C -1, respectively. How much heat will be required to change 150 grams of substance A from a solid at -40.0°C to a liquid at +70.0°C?
A)1.52 × 105 J
B)1.81 × 105 J
C)2.21 × 103 J
D)2.29 × 105 J
E)2.75 × 105 J
A)1.52 × 105 J
B)1.81 × 105 J
C)2.21 × 103 J
D)2.29 × 105 J
E)2.75 × 105 J

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
46
A solid with a molecular weight of 174.0 g/mol is at its melting point. If this solid requires 21.3 kJ of heat to melt 34.0 g, what is the molar heat of fusion of this solid (in kJ/mol)
A)3.60 × 10-3 kJ/mol
B)4.16 kJ/mol
C)2.40 × 10-1 kJ/mol
D)109 kJ/mol
E)1.26 × 105 kJ/mol
A)3.60 × 10-3 kJ/mol
B)4.16 kJ/mol
C)2.40 × 10-1 kJ/mol
D)109 kJ/mol
E)1.26 × 105 kJ/mol

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
47
A solid with a molecular weight of 148.0 g/mol is already at its melting point. If 18.6 g of this solid requires 4.838 kJ of heat to melt it, what is the molar heat of fusion (in kJ/mol)?
A)1.33 × 104 kJ/mol
B)0.608 kJ/mol
C)569 kJ/mol
D)0.0260 kJ/mol
E)38.4 kJ/mol
A)1.33 × 104 kJ/mol
B)0.608 kJ/mol
C)569 kJ/mol
D)0.0260 kJ/mol
E)38.4 kJ/mol

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
48
22.4 g of a solid with a molecular weight of 148.0 g/mol is at its melting point. If this solid requires 5.358 kJ of heat to melt it, what is the molar heat of fusion, in kJ/mol, for this solid?
A)1.23 × 104 kJ/mol
B)0.811 kJ/mol
C)35.4 kJ/mol
D)0.0282 kJ/mol
E)1.78 × 104 kJ/mol
A)1.23 × 104 kJ/mol
B)0.811 kJ/mol
C)35.4 kJ/mol
D)0.0282 kJ/mol
E)1.78 × 104 kJ/mol

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
49
Given the phase changes: condensation, freezing, fusion, sublimation, and vaporization. Which of these phase changes is/are endothermic?
A)condensation and freezing only
B)fusion only
C)fusion and vaporization only
D)fusion and sublimation only
E)sublimation, fusion, and vaporization only
A)condensation and freezing only
B)fusion only
C)fusion and vaporization only
D)fusion and sublimation only
E)sublimation, fusion, and vaporization only

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
50
Supercooling is defined as
A)the extremely rapid cooling of a vapor to form a liquid.
B)the use of extremely cold refrigerants to achieve smaller crystal size when liquids are frozen.
C)the extremely rapid cooling of a liquid to form a softer crystalline solid.
D)the cooling of a liquid to a temperature below its melting point without solidification.
E)the cooling of a substance to absolute zero.
A)the extremely rapid cooling of a vapor to form a liquid.
B)the use of extremely cold refrigerants to achieve smaller crystal size when liquids are frozen.
C)the extremely rapid cooling of a liquid to form a softer crystalline solid.
D)the cooling of a liquid to a temperature below its melting point without solidification.
E)the cooling of a substance to absolute zero.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds should have the largest value for heat of vaporization ( Hvap)?
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I
A)CH3-Br
B)CH3-Cl
C)CH3-F
D)CH3-H
E)CH3-I

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following compounds should have the largest value for heat of fusion ( Hfus)?
A)NH3
B)CH3-Cl
C)N2
D)HCl
A)NH3
B)CH3-Cl
C)N2
D)HCl

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
53
Dioxane, C4H8O2, has an enthalpy of fusion of 145.8 J g-1 and its melting point temperature is 11.0°C. How much heat is required to transform 142 g of solid dioxane, into liquid dioxane, at 11.0°C?
A)1.60 kJ
B)2.59 kJ
C)20.7 kJ
D)22.8 kJ
E)40.3 kJ
A)1.60 kJ
B)2.59 kJ
C)20.7 kJ
D)22.8 kJ
E)40.3 kJ

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
54
A substance, B, has a normal boiling point of +89.3°C, and a heat of vaporization of 260 J/g. How much heat is required to change 150 grams of B from a liquid at 89.3°C to a gas at 89.3°C?
A)5.2 × 102 J
B)3.9 × 104 J
C)4.4 × 105 J
D)1.1 × 105 J
E)1.6 × 105 J
A)5.2 × 102 J
B)3.9 × 104 J
C)4.4 × 105 J
D)1.1 × 105 J
E)1.6 × 105 J

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
55
Evaporation
A)is an endothermic process.
B)is an exothermic process.
C)involves breaking intermolecular forces.
D)decreases the temperature on the surface where it occurs.
E)A, C, and D
A)is an endothermic process.
B)is an exothermic process.
C)involves breaking intermolecular forces.
D)decreases the temperature on the surface where it occurs.
E)A, C, and D

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
56
An isobar is a line of constant pressure which runs parallel to the temperature axis on the phase diagram for a substance. As we vary temperature along an isobar that lies below the triple point, which process would never be observed?
A)decrease in volume
B)deposition
C)expansion
D)fusion
E)sublimation
A)decrease in volume
B)deposition
C)expansion
D)fusion
E)sublimation

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
57
The triple point of a substance is the temperature and pressure at which
A)all three physical states cease to exist.
B)sublimation, fusion, and condensation are taking place simultaneously.
C)the solid will always float on the liquid for all substances.
D)the vapor pressure of the liquid is higher than the vapor pressure of the solid.
E)the vapor pressure of the solid is higher than the vapor pressure of the liquid.
A)all three physical states cease to exist.
B)sublimation, fusion, and condensation are taking place simultaneously.
C)the solid will always float on the liquid for all substances.
D)the vapor pressure of the liquid is higher than the vapor pressure of the solid.
E)the vapor pressure of the solid is higher than the vapor pressure of the liquid.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
58
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 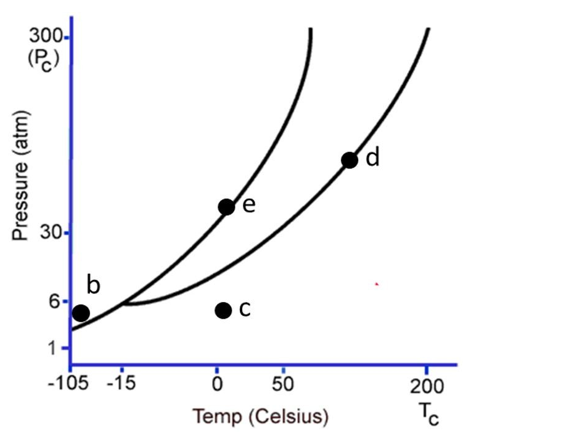
-At the temperature and pressure of point d, which statement below is true?
A)The substance will sublime.
B)There will be an equilibrium between the solid phase and the gaseous phase.
C)Vaporization and deposition will take place simultaneously.
D)Condensation and evaporation will take place simultaneously.
E)The substance will be a supercritical fluid.

-At the temperature and pressure of point d, which statement below is true?
A)The substance will sublime.
B)There will be an equilibrium between the solid phase and the gaseous phase.
C)Vaporization and deposition will take place simultaneously.
D)Condensation and evaporation will take place simultaneously.
E)The substance will be a supercritical fluid.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
59
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 
-At the temperature and pressure of point e, which statement below is true?
A)The substance will sublime.
B)There will be an equilibrium between the solid phase and the gaseous phase.
C)Vaporization and deposition will take place simultaneously.
D)Melting and freezing will take place simultaneously.
E)Melting and vaporization will take place simultaneously.

-At the temperature and pressure of point e, which statement below is true?
A)The substance will sublime.
B)There will be an equilibrium between the solid phase and the gaseous phase.
C)Vaporization and deposition will take place simultaneously.
D)Melting and freezing will take place simultaneously.
E)Melting and vaporization will take place simultaneously.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
60
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 
-Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
A)the substance will sublime.
B)the substance will undergo fusion.
C)the substance will undergo deposition.
D)the substance will freeze.
E)the substance will be a supercritical fluid.

-Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
A)the substance will sublime.
B)the substance will undergo fusion.
C)the substance will undergo deposition.
D)the substance will freeze.
E)the substance will be a supercritical fluid.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
61
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 
-Starting at the temperature and pressure of c, if the pressure is increased at constant temperature, ultimately
A)the substance will sublime.
B)the substance will undergo fusion.
C)the substance will undergo deposition.
D)the substance will freeze.
E)the substance will undergo condensation.

-Starting at the temperature and pressure of c, if the pressure is increased at constant temperature, ultimately
A)the substance will sublime.
B)the substance will undergo fusion.
C)the substance will undergo deposition.
D)the substance will freeze.
E)the substance will undergo condensation.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
62
The following questions refer to the phase diagram below. 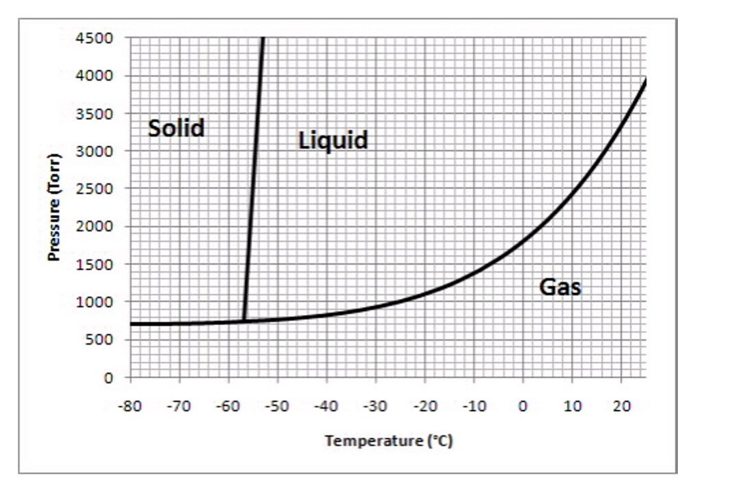
-What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

-What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
63
The following questions refer to the phase diagram below. 
-What phase should this substance exist as, at a pressure of 2000 torr and a temperature of 20°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

-What phase should this substance exist as, at a pressure of 2000 torr and a temperature of 20°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
64
The following questions refer to the phase diagram below. 
-What phase should this substance exist as, at a pressure of 2000 torr and a temperature of -70°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

-What phase should this substance exist as, at a pressure of 2000 torr and a temperature of -70°C?
A)solid
B)liquid
C)gas
D)supercritical fluid
E)unable to tell

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
65
The following questions refer to the phase diagram below. 
-Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
A)-21°C and 1000 torr
B)0°C and 1000 torr.
C)-57°C and 740 torr
D)-50°C and 4500 torr
E)0°C and 1760 torr

-Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
A)-21°C and 1000 torr
B)0°C and 1000 torr.
C)-57°C and 740 torr
D)-50°C and 4500 torr
E)0°C and 1760 torr

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
66
Which compound cannot be liquefied by compression, at a temperature of 25.0°C?
A)C2H4, critical point: 9.9°C, 50.5 atm
B)CH3Cl, critical point: 144.0°C, 66.0 atm
C)C2H2, critical point: 35.5°C, 61.6 atm
D)C2H6, critical point: 32.2°C, 48.2 atm
E)SO2, critical point: 158.0°C, 78.0 atm
A)C2H4, critical point: 9.9°C, 50.5 atm
B)CH3Cl, critical point: 144.0°C, 66.0 atm
C)C2H2, critical point: 35.5°C, 61.6 atm
D)C2H6, critical point: 32.2°C, 48.2 atm
E)SO2, critical point: 158.0°C, 78.0 atm

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
67
The critical temperature of a substance is
A)always higher than the triple point temperature.
B)the temperature below which it cannot exist in the liquid state.
C)the temperature which is always higher than the Kelvin point.
D)the temperature below which it cannot be liquefied by increasing the pressure.
E)the temperature below which it cannot be supercooled.
A)always higher than the triple point temperature.
B)the temperature below which it cannot exist in the liquid state.
C)the temperature which is always higher than the Kelvin point.
D)the temperature below which it cannot be liquefied by increasing the pressure.
E)the temperature below which it cannot be supercooled.

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
68
Which compound cannot be liquefied by compression, at a temperature of 25.0°C?
A)CH3Cl, critical point: 144.0°C, 66.0 atm
B)C2H2, critical point: 35.5°C, 61.6 atm
C)C2H6, critical point: 32.2°C, 48.2 atm
D)SiF4, critical point: -14.1°C, 36.7 atm
E)SO2, critical point: 158.0°C, 78.0 atm
A)CH3Cl, critical point: 144.0°C, 66.0 atm
B)C2H2, critical point: 35.5°C, 61.6 atm
C)C2H6, critical point: 32.2°C, 48.2 atm
D)SiF4, critical point: -14.1°C, 36.7 atm
E)SO2, critical point: 158.0°C, 78.0 atm

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
69
The normal boiling point of acetic acid, HC2H3O2, is 117.9°C, and its heat of vaporization is 39,690 J/mol. What is its vapor pressure at 100.0°C?
A)423.2 torr
B)479.7 torr
C)586.6 torr
D)616.2 torr
E)694.4 torr
A)423.2 torr
B)479.7 torr
C)586.6 torr
D)616.2 torr
E)694.4 torr

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
70
Find the boiling temperature at 760 torr of an isomer of octane, C8H18, if its heat of vaporization is 38,210 J mol-1 and its vapor pressure at 110.0°C is 638.43 torr.
A)111.52°C
B)113.22°C
C)115.00°C
D)115.65°C
E)118.30°C
A)111.52°C
B)113.22°C
C)115.00°C
D)115.65°C
E)118.30°C

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
71
Hexane (C6H14)boils at 68.73°C at 760 torr. The heat of vaporization for hexane is28.9 kJ/mol. What is the vapor pressure of hexane at 30°C?
A)207 torr
B)546 torr
C)2786 torr
D)759 torr
E)2396 torr
A)207 torr
B)546 torr
C)2786 torr
D)759 torr
E)2396 torr

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
72
The normal boiling point of 2,3,4-trimethypentane, C8H18, is 113.47°C, and its heat of vaporization is 37,600 J mol-1. What is its vapor pressure at 105.5°C?
A)479.7 torr
B)586.6 torr
C)594.1 torr
D)616.2 torr
E)694.4 torr
A)479.7 torr
B)586.6 torr
C)594.1 torr
D)616.2 torr
E)694.4 torr

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
73
Portions of how many different atoms are in one simple cubic unit cell?
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
74
Which type of lattice structures have lattice points on all of the faces?
A)simple cubic structures
B)base-centered structures
C)face-centered structures
D)body-centered structures
E)both body-centered and base-centered structures
A)simple cubic structures
B)base-centered structures
C)face-centered structures
D)body-centered structures
E)both body-centered and base-centered structures

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
75
Which type of unit cell has lattice points at each corner, and one in the center of the cell?
A)face-centered cubic
B)simple cubic
C)body-centered cubic
D)edge-centered cubic
E)hexagonal-centered cubic
A)face-centered cubic
B)simple cubic
C)body-centered cubic
D)edge-centered cubic
E)hexagonal-centered cubic

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
76
In a one-element solid with a face-centered cubic structure, how many atoms are in one unit cell?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
77
In a one-element solid with a simple cubic crystal structure, how many atoms are in one unit cell?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
78
A new compound, boganium sulfide, has been discovered. X-ray crystallographic studies reveal that it has a cubic unit cell with a sulfide ion at each of the corner lattice points, a sulfide ion at the geometric center of the unit cell, and a boganium ion in the center of each of the cube faces in the unit cell. Based on this structure, the formula for the compound should be
A)Bo3S
B)Bo3S2
C)BoS
D)Bo2S3
E)BoS3
A)Bo3S
B)Bo3S2
C)BoS
D)Bo2S3
E)BoS3

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
79
What type of crystal lattice is found in a sample of table salt, NaCl?
A)simple cubic
B)base-centered cubic
C)body-centered cubic
D)face-centered cubic
E)orthorhombic-centered cubic
A)simple cubic
B)base-centered cubic
C)body-centered cubic
D)face-centered cubic
E)orthorhombic-centered cubic

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck
80
How many atoms are contained in one unit cell of metallic tungsten, if it forms a face-centered cubic unit cell?
A)2
B)4
C)8
D)9
E)14
A)2
B)4
C)8
D)9
E)14

Unlock Deck
Unlock for access to all 189 flashcards in this deck.
Unlock Deck
k this deck


