Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
A substance has a normal boiling point of +89.3 °C, an enthalpy of vaporization of 2600 J g-1, and specific heats for the liquid and the gas are 6.20 and 3.20 J g-1 °C -1, respectively. How much energy is needed to change 150 grams of the substance from a liquid at -10.0 °C to a gas at +129.0 °C?
Free
(Short Answer)
4.9/5  (34)
(34)
Correct Answer:
5.01 × 105 J or 501 kJ
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 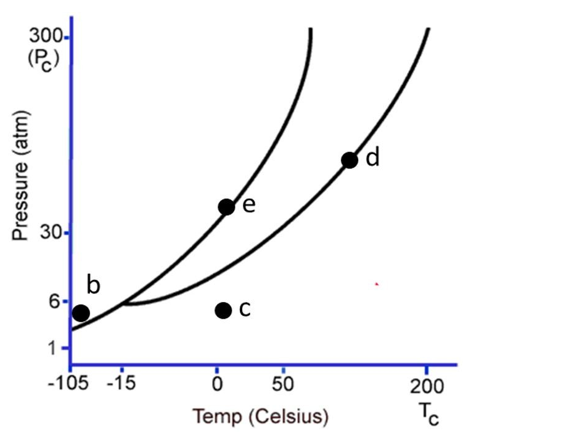 -At the temperature and pressure of point e, which statement below is true?
-At the temperature and pressure of point e, which statement below is true?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
D
Keshon has an equation that gives the relationship between the air pressure at sea level, p0, and the air pressure, p, at a height, h (in meters), above sea level:h = 4405 ln 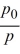 He found a value for ΔΗvap of water, 41,108 J mol-1. Keshon has now challenged two other whiz kids in the class with this problem: Their weather app has given the barometric pressure at sea level as 30.18 inches of mercury. What is the temperature of the boiling water in a teapot at a mountain camp which is 2200 meters (about 7220 feet)above sea level?Hint: Use the Clausius-Clapeyron equation and pay attention to units.
He found a value for ΔΗvap of water, 41,108 J mol-1. Keshon has now challenged two other whiz kids in the class with this problem: Their weather app has given the barometric pressure at sea level as 30.18 inches of mercury. What is the temperature of the boiling water in a teapot at a mountain camp which is 2200 meters (about 7220 feet)above sea level?Hint: Use the Clausius-Clapeyron equation and pay attention to units.
Free
(Short Answer)
4.9/5  (37)
(37)
Correct Answer:
86.7°C
Determine the shift in equilibrium that would result if 100 kJ of energy were added to ice melting at 0°C to form water (H2O(s)⇆ H2O(l)).
(Short Answer)
4.9/5  (27)
(27)
How many atoms are contained in one unit cell of metallic polonium, if it forms a simple cubic unit cell?
(Multiple Choice)
4.9/5  (36)
(36)
X-ray radiation at a wavelength of 1.542 × 10-10 m is used to irradiate a metallic crystal. The X-rays are being reflected at an angle of 12.73°. If this reflection is a first order reflection(n = 1), what is the distance between the planes of atoms in this metallic crystal?
(Multiple Choice)
4.8/5  (42)
(42)
A new compound, voronium oxide, has been discovered. X-ray crystallographic studies reveal that it has a cubic unit cell with a voronium ion at each of the corner lattice points and an oxide ion in the geometric center of the unit cell. Based on this structure, the formula for the compound should be
(Multiple Choice)
4.8/5  (41)
(41)
Given the following substances and their normal boiling points, in °C: 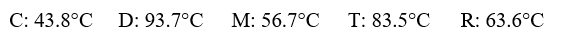 Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?
Which ranking correctly lists some of these liquids in order of decreasing intermolecular forces at 20°C?
(Multiple Choice)
4.8/5  (44)
(44)
Which one of the following substances is most likely composed of ions rather than distinct formula units, when it is in the solid state?
(Multiple Choice)
4.8/5  (39)
(39)
Arrange these compounds in order of increasing vapor pressure at 20°C: CCl4, GeCl4, SiCl4, SnCl4.
(Short Answer)
4.9/5  (39)
(39)
Given the following substances and their normal boiling points, in °C:  Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?
Which ranking correctly lists some of these substances in order of increasing vapor pressure at 20°C?
(Multiple Choice)
4.7/5  (30)
(30)
At 1.0 atm pressure, ice (solid H2O)floats in water instead of sinking. The reason for this is
(Multiple Choice)
4.7/5  (32)
(32)
An unknown solid is soft and lustrous. It has a low melting point and conducts electricity well as a solid or liquid. What kind of crystal best describes this solid?
(Multiple Choice)
4.7/5  (36)
(36)
The following questions refer to the phase diagram below. 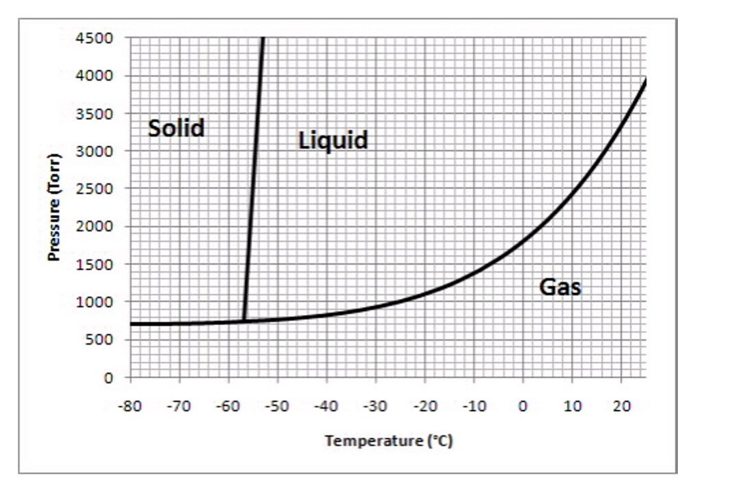 -Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
-Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
(Multiple Choice)
4.7/5  (32)
(32)
Which covalent compound will exhibit hydrogen bonding in the liquid state?
(Multiple Choice)
4.7/5  (43)
(43)
Solid carbon dioxide never forms a liquid at a pressure of one atmosphere, instead, it sublimes when left open to the atmosphere. Why?
(Short Answer)
4.7/5  (37)
(37)
The following questions refer to the diagram below. 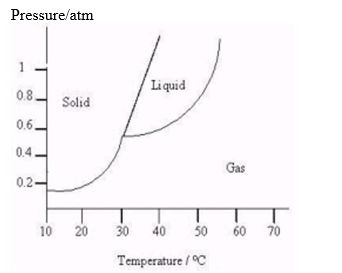 -How does the boiling point of this substance vary as the pressure on this substance increases?
-How does the boiling point of this substance vary as the pressure on this substance increases?
(Short Answer)
4.9/5  (36)
(36)
Which of the following liquids, at the same temperature, has the highest vapor pressure?
(Multiple Choice)
4.9/5  (38)
(38)
In a one-element solid with a simple cubic crystal structure, how many atoms are in one unit cell?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 189
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)