Deck 1: Elements Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/39
Play
Full screen (f)
Deck 1: Elements Compounds
1
Which of the following correctly describes one of the differences between compounds and mixtures?
A) Compounds contain more than one element but mixtures do not.
B) Compounds have a constant composition but mixtures do not.
C) It is easier to separate the elements in a compound than it is to separate the elements in a mixture.
D) All of these statements are true.
E) None of these statements are true.
A) Compounds contain more than one element but mixtures do not.
B) Compounds have a constant composition but mixtures do not.
C) It is easier to separate the elements in a compound than it is to separate the elements in a mixture.
D) All of these statements are true.
E) None of these statements are true.
Compounds have a constant composition but mixtures do not.
2
Which of the following postulates of Dalton's atomic theory is/are still valid today?
(I) Matter consists of particles called atoms.
(II) Atoms are indestructible and indivisible.
(III) All atoms of an element are identical.
(IV) Atoms of different elements differ in mass.
(V) When the atoms of different elements combine to form compounds they combine in simple whole number ratios.
A) All of these statements are still valid today.
B) I and II are still valid, but not III, IV and V.
C) I, II and III are still valid, but not IV and V.
D) I, IV and V are still valid, but not II and III.
E) II and III are still valid, but not I, IV and V.
(I) Matter consists of particles called atoms.
(II) Atoms are indestructible and indivisible.
(III) All atoms of an element are identical.
(IV) Atoms of different elements differ in mass.
(V) When the atoms of different elements combine to form compounds they combine in simple whole number ratios.
A) All of these statements are still valid today.
B) I and II are still valid, but not III, IV and V.
C) I, II and III are still valid, but not IV and V.
D) I, IV and V are still valid, but not II and III.
E) II and III are still valid, but not I, IV and V.
I, IV and V are still valid, but not II and III.
3
Calculate the number of protons in the average potassium atom if the atomic weight of potassium is 39 amu and the atomic number of this element is 19.
A) 19
B) 20
C) 39
D) 58
E) The number of protons is impossible to determine from this information.
A) 19
B) 20
C) 39
D) 58
E) The number of protons is impossible to determine from this information.
19
4
The earth is constantly bombarded by cosmic rays emitted by the sun. The total energy received in the form of cosmic rays is small - no more than the energy received by the planet from starlight. But the energy of a single cosmic ray is very large, on the order of 200 million kJ/mol. These highly energetic rays react with atoms in the atmosphere to produce neutrons that then react with nitrogen atoms in the atmosphere to produce 14C, which decays back to 14N with a half-life of 5730 years by emitting an electron.
What is the difference between 14C and 14N atoms?
A) They have the same number of protons but different numbers of neutrons.
B) They have the same number of neutrons but different numbers of protons.
C) They have the same number of neutrons and protons but different numbers of electrons.
D) 14N has one more proton and one less neutron than 14C.
E) 14N has one more neutron and one less proton than 14C.
What is the difference between 14C and 14N atoms?
A) They have the same number of protons but different numbers of neutrons.
B) They have the same number of neutrons but different numbers of protons.
C) They have the same number of neutrons and protons but different numbers of electrons.
D) 14N has one more proton and one less neutron than 14C.
E) 14N has one more neutron and one less proton than 14C.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following atoms have the same number of neutrons?
(I) 233Th (II) 235U (III) 238U (IV) 238Np
A) I and II
B) I, II and IV
C) II and III
D) III and IV
E) All of these atoms have the same number of neutrons.
(I) 233Th (II) 235U (III) 238U (IV) 238Np
A) I and II
B) I, II and IV
C) II and III
D) III and IV
E) All of these atoms have the same number of neutrons.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement correctly describes the difference between the 12C, 13C and 14C isotopes of carbon?
A) They have the same number of protons but different numbers of electrons.
B) They have the same number of neutrons but different numbers of electrons.
C) They have the same number of electrons but different numbers of protons.
D) They have the same number of protons but different numbers of neutrons.
E) They have the same number of protons, electrons, and neutrons but
Different atomic masses.
A) They have the same number of protons but different numbers of electrons.
B) They have the same number of neutrons but different numbers of electrons.
C) They have the same number of electrons but different numbers of protons.
D) They have the same number of protons but different numbers of neutrons.
E) They have the same number of protons, electrons, and neutrons but
Different atomic masses.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
7
What is the symbol for the atom or ion that has 34 neutrons, 29 protons, and 27 electrons?
A) 34Se2 -
B) 29Cu2+
C) 63Se2 -
D) 63Cu2+
E) 27Co2+
A) 34Se2 -
B) 29Cu2+
C) 63Se2 -
D) 63Cu2+
E) 27Co2+

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
8
How many electrons are in 52Cr3+?
A) 3
B) 21
C) 24
D) 27
E) 28
A) 3
B) 21
C) 24
D) 27
E) 28

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
9
How many protons are in 32S2-?
A) 2
B) 14
C) 16
D) 18
E) 32
A) 2
B) 14
C) 16
D) 18
E) 32

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
10
Complete the following table.
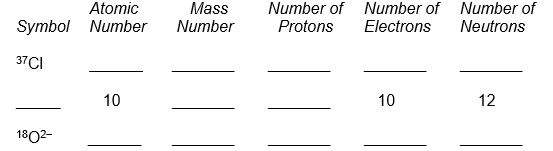


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
11
Complete the following table



Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
12
Complete the table
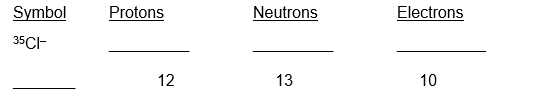
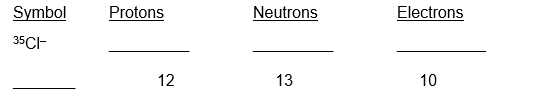

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
13
Describe the difference on the atomic scale between the following three forms of oxygen:
O, O2, O2 - .
O, O2, O2 - .

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
14
Which choice gives the diagrams in the order that best represents 2O2, 4O, and X3, respectively?
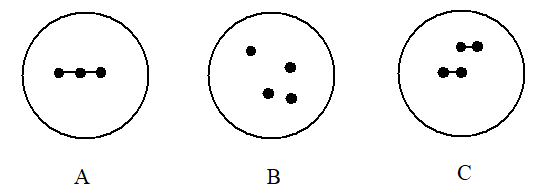
A) A B C
B) C B A
C) B C A
D) B A C
E) None of the above gives the cartoons in the correct order.

A) A B C
B) C B A
C) B C A
D) B A C
E) None of the above gives the cartoons in the correct order.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
15
Atoms are isobaric if they have the same mass. Which of the following atoms or ions are isobaric?
(I) 51Cr3+ (II) 52Cr3+ (III) 55Mn3+ (IV) 59Ni4+ (V) 59Co3+
A) I and II
B) II and III
C) III and IV
D) IV and V
E) III, IV and V
(I) 51Cr3+ (II) 52Cr3+ (III) 55Mn3+ (IV) 59Ni4+ (V) 59Co3+
A) I and II
B) II and III
C) III and IV
D) IV and V
E) III, IV and V

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following correctly describes a Zn2+ ion that has a mass number of 65?
A) It contains 28 electrons, 30 protons, and 35 neutrons.
B) It contains 30 electrons, 30 protons, and 35 neutrons.
C) It contains 32 electrons, 30 protons, and 35 neutrons.
D) It contains 35 electrons, 35 protons, and 30 neutrons.
E) It contains 37 electrons, 35 protons, and 30 neutrons.
A) It contains 28 electrons, 30 protons, and 35 neutrons.
B) It contains 30 electrons, 30 protons, and 35 neutrons.
C) It contains 32 electrons, 30 protons, and 35 neutrons.
D) It contains 35 electrons, 35 protons, and 30 neutrons.
E) It contains 37 electrons, 35 protons, and 30 neutrons.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the number of electrons in a P3- ion if the mass number of phosphorus is 31 and the atomic number of this element is 15.
A) 12
B) 15
C) 18
D) 31
E) 34
A) 12
B) 15
C) 18
D) 31
E) 34

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following symbols indicates an atom or ion with 12 protons and 10 electrons?
A) Mg2+
B) C4+
C) O2-
D) Ne
E) none of these
A) Mg2+
B) C4+
C) O2-
D) Ne
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
19
All of the following have the same number of electrons except:
A) K+
B) Ca2+
C) Al3+
D) S2-
E) Cl-
A) K+
B) Ca2+
C) Al3+
D) S2-
E) Cl-

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
20
What is the symbol for the atom or ion that has 26 protons, 30 neutrons, and 24Electrons?
A) 56Cr2+
B) 54Cr2+
C) 56Fe2+
D) 54Fe3+
E) 54Cr
A) 56Cr2+
B) 54Cr2+
C) 56Fe2+
D) 54Fe3+
E) 54Cr

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
21
How many electrons are present in the 77Se2- ion?
A) 32
B) 34
C) 43
D) 77
E) none of these
A) 32
B) 34
C) 43
D) 77
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
22
How many electrons are present in the 136Ba2+ ion?
A) 56
B) 58
C) 78
D) 134
E) none of these
A) 56
B) 58
C) 78
D) 134
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the number of electrons on a Ce4+ ion if the atomic number of this
Element is 58 and the mass number of the ion is 140.
A) 54
B) 58
C) 62
D) 136
E) 140
Element is 58 and the mass number of the ion is 140.
A) 54
B) 58
C) 62
D) 136
E) 140

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds is composed only of polyatomic ions?
A) NH4NO3
B) CaO
C) H2SO4
D) NO2
E) CO2
A) NH4NO3
B) CaO
C) H2SO4
D) NO2
E) CO2

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a nonmetal in period 4?
A) C
B) Ca
C) Se
D) Sr
E) none of these
A) C
B) Ca
C) Se
D) Sr
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following elements is neither a metal nor a nonmetal?
A) Na
B) Mg
C) Ga
D) Si
E) P
A) Na
B) Mg
C) Ga
D) Si
E) P

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
27
Classify the following elements as metals, nonmetals or semi-metals:
S, Sb, Sc, Se, Sg, Si, Sm, Sn, and Sr.
S, Sb, Sc, Se, Sg, Si, Sm, Sn, and Sr.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
28
use the data listed below.

-If you were able to select a single atom of oxygen from a naturally occurring sample, what would its mass most likely be?
A) 15.9949 amu
B) 16.9991 amu
C) 17.9992 amu
D) 15.9993 amu
E) none of these

-If you were able to select a single atom of oxygen from a naturally occurring sample, what would its mass most likely be?
A) 15.9949 amu
B) 16.9991 amu
C) 17.9992 amu
D) 15.9993 amu
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
29
use the data listed below.

-If you were able to select 1000 individual atoms of oxygen from a naturally occurring sample, what would be the mass of the atoms?
A) 15.9949 amu
B) 16.9991 amu
C) 17.9992 amu
D) 15.9993 amu
E) none of these

-If you were able to select 1000 individual atoms of oxygen from a naturally occurring sample, what would be the mass of the atoms?
A) 15.9949 amu
B) 16.9991 amu
C) 17.9992 amu
D) 15.9993 amu
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
30
use the data listed below.

-If you select a random atom of Ne from a naturally occurring sample, its mass is most likely to be
A) 19.99244 amu
B) 20.99384 amu
C) 21.99138 amu
D) 20.179 amu
E) 20.000 amu

-If you select a random atom of Ne from a naturally occurring sample, its mass is most likely to be
A) 19.99244 amu
B) 20.99384 amu
C) 21.99138 amu
D) 20.179 amu
E) 20.000 amu

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
31
use the data listed below.

-If you select random atoms of Ne from a naturally occurring sample, their mass is most likely to be
A) 1999.244 amu
B) 2099.384 amu
C) 2199.138 amu
D) 2017.9 amu
E) 2000.0 amu

-If you select random atoms of Ne from a naturally occurring sample, their mass is most likely to be
A) 1999.244 amu
B) 2099.384 amu
C) 2199.138 amu
D) 2017.9 amu
E) 2000.0 amu

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
32
What will be the coefficient for HNO3 when the following equation is balanced?
Ba(OH)2(aq) + HNO3(aq) Ba(NO3)2(aq) + H2O(l)
A) 1
B) 2
C) 3
D) 5
E) none of these
Ba(OH)2(aq) + HNO3(aq) Ba(NO3)2(aq) + H2O(l)
A) 1
B) 2
C) 3
D) 5
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
33
What will be the coefficient for oxygen when the following equation is balanced?
C3H8(g) + O2(g) CO2(g) + H2O(g)
A) 1
B) 2
C) 3
D) 5
E) none of these
C3H8(g) + O2(g) CO2(g) + H2O(g)
A) 1
B) 2
C) 3
D) 5
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
34
What will be the coefficient for NH3 when the following reaction is balanced?
NH3(g) + O2(g) N2(g) + H2O(l)
A) 1
B) 3
C) 4
D) 5
E) none of these
NH3(g) + O2(g) N2(g) + H2O(l)
A) 1
B) 3
C) 4
D) 5
E) none of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
35
Balance the following equations:
(I) Na2SO4(s) + C(s) Na2S(aq) + CO2(g)
(II) Mg3N2(s) + H2O(l) Mg(OH)2(aq) + NH3(g)
(I) Na2SO4(s) + C(s) Na2S(aq) + CO2(g)
(II) Mg3N2(s) + H2O(l) Mg(OH)2(aq) + NH3(g)

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
36
The balanced equation for the decomposition of ammonium dichromate has which of the following sets of coefficients?
A (NH4)2Cr2O7(s) B Cr2O3(s) + C N2(g) + D H2O(g)
A) A = 2, B = 2, C = 2, D = 4
B) A = 1, B = 1, C = 1, D = 4
C) A = 1, B = 1, C = 1, D = 2
D) A = 1, B = 1, C = 2, D = 2
E) A = B = C = D = 1
A (NH4)2Cr2O7(s) B Cr2O3(s) + C N2(g) + D H2O(g)
A) A = 2, B = 2, C = 2, D = 4
B) A = 1, B = 1, C = 1, D = 4
C) A = 1, B = 1, C = 1, D = 2
D) A = 1, B = 1, C = 2, D = 2
E) A = B = C = D = 1

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the sum of the coefficients in the balanced chemical equation for the following reaction:
A H2S(g) + B O2(g) C SO2(g) + D H2O(g)
A) 6
B) 8
C) 9
D) 11
E) 17
A H2S(g) + B O2(g) C SO2(g) + D H2O(g)
A) 6
B) 8
C) 9
D) 11
E) 17

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
38
What is the sum of the coefficients when the following chemical equation is balanced?
A Ca3(PO4)2(s) + B C(s) C Ca3P2(s) + D CO(g)
A) 6
B) 12
C) 18
D) 20
E) none of the above
A Ca3(PO4)2(s) + B C(s) C Ca3P2(s) + D CO(g)
A) 6
B) 12
C) 18
D) 20
E) none of the above

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
39
Which illustration best represents the following equation?

A)
B)
C)
D)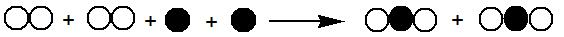
E)

A)

B)

C)

D)
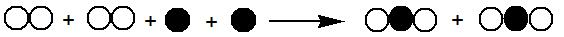
E)


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck


