Exam 1: Elements Compounds
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following symbols indicates an atom or ion with 12 protons and 10 electrons?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
A
All of the following have the same number of electrons except:
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
use the data listed below.
 -If you were able to select a single atom of oxygen from a naturally occurring sample, what would its mass most likely be?
-If you were able to select a single atom of oxygen from a naturally occurring sample, what would its mass most likely be?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
A
Calculate the number of protons in the average potassium atom if the atomic weight of potassium is 39 amu and the atomic number of this element is 19.
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the sum of the coefficients in the balanced chemical equation for the following reaction:
A H2S(g) + B O2(g) C SO2(g) + D H2O(g)
(Multiple Choice)
4.8/5  (43)
(43)
The earth is constantly bombarded by cosmic rays emitted by the sun. The total energy received in the form of cosmic rays is small - no more than the energy received by the planet from starlight. But the energy of a single cosmic ray is very large, on the order of 200 million kJ/mol. These highly energetic rays react with atoms in the atmosphere to produce neutrons that then react with nitrogen atoms in the atmosphere to produce 14C, which decays back to 14N with a half-life of 5730 years by emitting an electron.
What is the difference between 14C and 14N atoms?
(Multiple Choice)
4.8/5  (33)
(33)
What is the sum of the coefficients when the following chemical equation is balanced?
A Ca3(PO4)2(s) + B C(s) C Ca3P2(s) + D CO(g)
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following compounds is composed only of polyatomic ions?
(Multiple Choice)
4.7/5  (32)
(32)
Describe the difference on the atomic scale between the following three forms of oxygen:
O, O2, O2 - .
(Essay)
4.9/5  (42)
(42)
Which of the following atoms have the same number of neutrons?
(I) 233Th (II) 235U (III) 238U (IV) 238Np
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following elements is neither a metal nor a nonmetal?
(Multiple Choice)
4.8/5  (40)
(40)
The balanced equation for the decomposition of ammonium dichromate has which of the following sets of coefficients?
A (NH4)2Cr2O7(s) B Cr2O3(s) + C N2(g) + D H2O(g)
(Multiple Choice)
4.7/5  (27)
(27)
Balance the following equations:
(I) Na2SO4(s) + C(s) Na2S(aq) + CO2(g)
(II) Mg3N2(s) + H2O(l) Mg(OH)2(aq) + NH3(g)
(Essay)
4.9/5  (37)
(37)
Which of the following postulates of Dalton's atomic theory is/are still valid today?
(I) Matter consists of particles called atoms.
(II) Atoms are indestructible and indivisible.
(III) All atoms of an element are identical.
(IV) Atoms of different elements differ in mass.
(V) When the atoms of different elements combine to form compounds they combine in simple whole number ratios.
(Multiple Choice)
4.9/5  (36)
(36)
Which statement correctly describes the difference between the 12C, 13C and 14C isotopes of carbon?
(Multiple Choice)
4.8/5  (31)
(31)
Which choice gives the diagrams in the order that best represents 2O2, 4O, and X3, respectively?
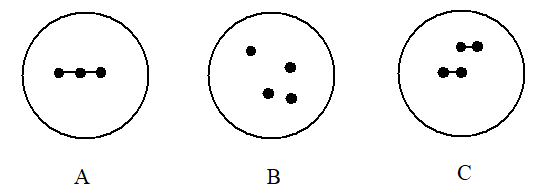
(Multiple Choice)
4.8/5  (35)
(35)
What will be the coefficient for oxygen when the following equation is balanced?
C3H8(g) + O2(g) CO2(g) + H2O(g)
(Multiple Choice)
4.9/5  (41)
(41)
Showing 1 - 20 of 39
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)