Deck 13: Thin Layer Chromatography
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 13: Thin Layer Chromatography
1
Calculate the Rf values for the following compounds.
(a) Spot, 5.0 cm; solvent front, 20.0 cm
(b) Spot, 3.0 cm; solvent front, 12.0 cm
(c) Spot, 9.8 cm; solvent front, 12.0 cm
(a) Spot, 5.0 cm; solvent front, 20.0 cm
(b) Spot, 3.0 cm; solvent front, 12.0 cm
(c) Spot, 9.8 cm; solvent front, 12.0 cm
(a) Rf = 5.0 cm / 20.0 cm = 0.25
(b) 0.25
(c) 0.82
(b) 0.25
(c) 0.82
2
If two compounds have Rf values of 0.50 and 0.61, how far will they be separated from each other on a plate when the solvent front is developed to
(a) 5 cm?
(b) 15 cm?
(a) 5 cm?
(b) 15 cm?
(a) x cm / 5.0 cm = 0.50; x = 2.5 cm. Similarly, y = 3.05 cm. Separation = 3.05 - 2.5) = 0.55 cm.
(b) 1.65 cm
(b) 1.65 cm
3
A wick of filter paper is placed in a TLC developing jar, and the atmosphere in the jar is satu- rated with solvent before a plate is developed. What would happen if a plate were developed in a jar with an atmosphere not saturated with solvent vapor?
The solvent front would not move evenly up the plate because it would be evaporating from the plate. Also the Rf from such a plate would be unreliable because more solvent is moving up the plate than normal, moving the spot farther up the plate than it should be.
4
A student spots an unknown sample on a TLC plate. A single spot with an Rf of 0.55 showed up on the plate after developing the sample in hexanes-ethyl acetate 50:50. Does this indicate that the unknown material is a pure compound? What can be done to verify the purity of the sample?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
5 As a separation and detection method, would TLC or paper chromatography yield better results in the analysis of each of the following pairs of compounds?
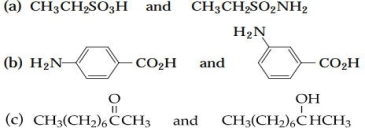
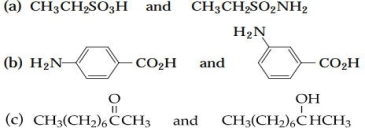

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Consider a sample that is a mixture composed of biphenyl, benzoic acid, and benzyl alcohol. The sample is spotted on a TLC plate and developed in a hexanes-ethyl acetate solvent mix- ture. Predict the relative Rf values for the three compounds in the sample.
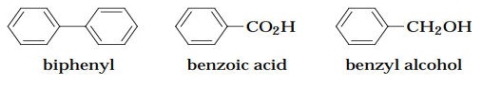


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
After a rather lengthy organic chemistry synthesis procedure, a student ran the product of the reaction on a TLC plate and obtained the result below. What might he / she have done wrong, if anything?


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
A student is following a reaction (the conversion of compound A to Compound B) by TLC. Aliquots of the reaction mixture are taken and analyzed at time = 0, 10, 20, and 30 min. The TLC plate, developed in 90% hexanes-10% ethyl acetate, is shown below.
(a) Which is the more polar compound, A or B?
(b) Is the reaction complete at 10 min? 20 min? 30 min?
(c) How can the identity of Compound B be verified by TLC?
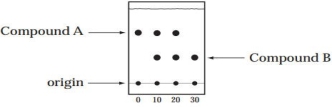
(a) Which is the more polar compound, A or B?
(b) Is the reaction complete at 10 min? 20 min? 30 min?
(c) How can the identity of Compound B be verified by TLC?


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the TLC plate illustrated in Problem 8. Would the Rf of Compound A be different if the solvent system used to develop the plate was 50% hexanes-50% ethyl acetate? If so, would it be lower or higher?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Again consider the TLC plate illustrated in Problem 12.8. Would the Rf of Compound B be dif- ferent if the solvent system used to develop the plate was 100% hexanes? If so, would it be lower or higher?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


