Exam 13: Thin Layer Chromatography
Exam 1: Introduction to the Organic Laboratory16 Questions
Exam 2: Crystallization11 Questions
Exam 3: Melting Points10 Questions
Exam 4: Extraction14 Questions
Exam 5: Drying Organic Solutions6 Questions
Exam 6: Simple Distillation10 Questions
Exam 7: Fractional Distillation8 Questions
Exam 8: Vacuum Distillation6 Questions
Exam 9: Steam Distillation6 Questions
Exam 10: Sublimation4 Questions
Exam 11: Refractive Index3 Questions
Exam 12: Column Chromatography7 Questions
Exam 13: Thin Layer Chromatography10 Questions
Exam 14: Gas Chromatography10 Questions
Exam 15: Carrying Out Typical Reactions8 Questions
Exam 16: Infrared Spectroscopy6 Questions
Exam 17: Proton Nuclear Magnetic Resonance Spectroscopy8 Questions
Exam 18: The Chemical Literature8 Questions
Select questions type
5 As a separation and detection method, would TLC or paper chromatography yield better results in the analysis of each of the following pairs of compounds?
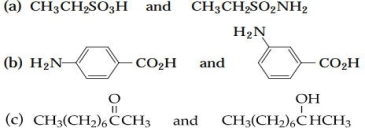
Free
(Essay)
4.8/5  (47)
(47)
Correct Answer:
(a) very polar: paper
(b) very polar: paper
(c) less polar: TLC
Consider the TLC plate illustrated in Problem 8. Would the Rf of Compound A be different if the solvent system used to develop the plate was 50% hexanes-50% ethyl acetate? If so, would it be lower or higher?
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
Yes, the Rf of Compound A be different. 50% Hexanes-50% ethyl acetate is a more polar sol- vent system than is 90% hexanes-10% ethyl acetate. Therefore, the Rf of Compound A would be greater because the compound will travel further up the plate.
After a rather lengthy organic chemistry synthesis procedure, a student ran the product of the reaction on a TLC plate and obtained the result below. What might he / she have done wrong, if anything?
Free
(Essay)
4.7/5  (42)
(42)
Correct Answer:
Either too much compound was spotted or the sample is very impure. The student should dilute the sample and re-run the TLC. If a streak is again obtained, the student's product is impure.
A student spots an unknown sample on a TLC plate. A single spot with an Rf of 0.55 showed up on the plate after developing the sample in hexanes-ethyl acetate 50:50. Does this indicate that the unknown material is a pure compound? What can be done to verify the purity of the sample?
(Essay)
4.8/5  (37)
(37)
If two compounds have Rf values of 0.50 and 0.61, how far will they be separated from each other on a plate when the solvent front is developed to
(a) 5 cm?
(b) 15 cm?
(Essay)
4.9/5  (40)
(40)
Calculate the Rf values for the following compounds.
(a) Spot, 5.0 cm; solvent front, 20.0 cm
(b) Spot, 3.0 cm; solvent front, 12.0 cm
(c) Spot, 9.8 cm; solvent front, 12.0 cm
(Essay)
4.7/5  (31)
(31)
A wick of filter paper is placed in a TLC developing jar, and the atmosphere in the jar is satu- rated with solvent before a plate is developed. What would happen if a plate were developed in a jar with an atmosphere not saturated with solvent vapor?
(Essay)
5.0/5  (36)
(36)
Again consider the TLC plate illustrated in Problem 12.8. Would the Rf of Compound B be dif- ferent if the solvent system used to develop the plate was 100% hexanes? If so, would it be lower or higher?
(Essay)
4.9/5  (38)
(38)
Consider a sample that is a mixture composed of biphenyl, benzoic acid, and benzyl alcohol. The sample is spotted on a TLC plate and developed in a hexanes-ethyl acetate solvent mix- ture. Predict the relative Rf values for the three compounds in the sample.
(Essay)
4.8/5  (48)
(48)
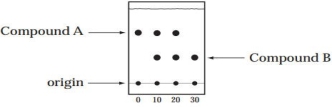
A student is following a reaction (the conversion of compound A to Compound B) by TLC. Aliquots of the reaction mixture are taken and analyzed at time = 0, 10, 20, and 30 min. The TLC plate, developed in 90% hexanes-10% ethyl acetate, is shown below.
(a) Which is the more polar compound, A or B?
(b) Is the reaction complete at 10 min? 20 min? 30 min?
(c) How can the identity of Compound B be verified by TLC?
(Essay)
4.9/5  (43)
(43)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)