Deck 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry
1
Which estimates the number of electrons per cubic centimeter excited to the conduction band as a function of temperature and the band gap energy. 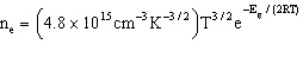
-Refer to Exhibit 22-2. Based solely on the equation above, which set of conditions will result in the most electrons being excited to the conduction band.
A) Low temperature, low band gap energy
B) Low temperature, high band gap energy
C) High temperature, low band gap energy
D) High temperature, high band gap energy
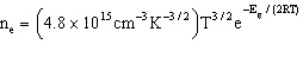
-Refer to Exhibit 22-2. Based solely on the equation above, which set of conditions will result in the most electrons being excited to the conduction band.
A) Low temperature, low band gap energy
B) Low temperature, high band gap energy
C) High temperature, low band gap energy
D) High temperature, high band gap energy
High temperature, low band gap energy
2
Which estimates the number of electrons per cubic centimeter excited to the conduction band as a function of temperature and the band gap energy. 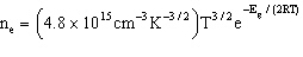
-Refer to Exhibit 22-2. The band gap of pure crystalline silicon is 1.94´10-19 J at 300 K. How many electrons are excited from the valence band to the conduction band at this temperature in a 1.00 cm3 silicon crystal?
A) 1.70´109
B) 4.80´1015
C) 0.555 NA
D) 1.46´107
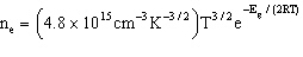
-Refer to Exhibit 22-2. The band gap of pure crystalline silicon is 1.94´10-19 J at 300 K. How many electrons are excited from the valence band to the conduction band at this temperature in a 1.00 cm3 silicon crystal?
A) 1.70´109
B) 4.80´1015
C) 0.555 NA
D) 1.46´107
1.70´109
3
Which of the following is not an acceptable location for an atom in a side-centered primitive cell?
A) (1,1,0)
B) (1,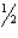 ,0)
,0)
C)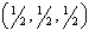
D) all of the above
E) none of the above
A) (1,1,0)
B) (1,
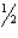 ,0)
,0)C)
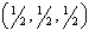
D) all of the above
E) none of the above
all of the above
4
An element crystallizes in a body-centered cubic lattice, with a = 4.109 Å. If the element has a density of 9.95 g·cm-3, what is the molar mass of the element?
A) 104 g·mol-1
B) 208 g·mol-1
C) 416 g·mol-1
D) 831 g·mol-1
A) 104 g·mol-1
B) 208 g·mol-1
C) 416 g·mol-1
D) 831 g·mol-1

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
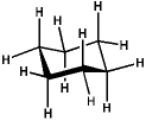
Which of the following will have the maximum ultraviolet absorbance at the longest wavelength?
A)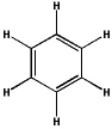
B)
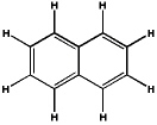
C)
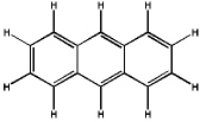
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following symbols also represents a b particle?
A)
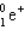
B)
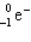
C)
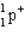
D)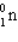
E) None of the above
A)
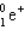
B)
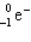
C)
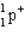
D)
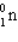
E) None of the above

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the value of the decay constant k?
A) 7.00´10-2 d-1
B) 2.11´10-2 d-1
C) 4.85´10-2 d-1
D) 9.70´10-2 d-1
-Refer to Exhibit 19-1. What is the value of the decay constant k?
A) 7.00´10-2 d-1
B) 2.11´10-2 d-1
C) 4.85´10-2 d-1
D) 9.70´10-2 d-1

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample initially?
A) 1.07´1016 Bq
B) 2.68´1015 Bq
C) 4.28´1016 Bq
D) 5.33´1015 Bq
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample initially?
A) 1.07´1016 Bq
B) 2.68´1015 Bq
C) 4.28´1016 Bq
D) 5.33´1015 Bq

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample after 30 days?
A) 6.26´1014 Bq
B) 2.50´1015 Bq
C) 1.42´1015 Bq
D) 1.42´1014 Bq
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample after 30 days?
A) 6.26´1014 Bq
B) 2.50´1015 Bq
C) 1.42´1015 Bq
D) 1.42´1014 Bq

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. How long will it take for the activity of the 0.25 g sample to fall below 1 Ci? (1 Ci=3.7´1010 Bq)
A) 190 days
B) 217 days
C) 231 days
D) 259 days
-Refer to Exhibit 19-1. How long will it take for the activity of the 0.25 g sample to fall below 1 Ci? (1 Ci=3.7´1010 Bq)
A) 190 days
B) 217 days
C) 231 days
D) 259 days

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
pertain to a 450 gram sample of 60Co recovered from a decommissioned nuclear reactor. An activity of 1.11´1013 Bq was measured by technicians dismantling the plant.
-Refer to Exhibit 19-2. The activity of the sample was measured a year later and found to be 9.73´1012 Bq. What is the decay constant?
A) 0.132 d-1
B) 0.0572 d-1
C) 3.12 d-1
D) 0.319 d-1
-Refer to Exhibit 19-2. The activity of the sample was measured a year later and found to be 9.73´1012 Bq. What is the decay constant?
A) 0.132 d-1
B) 0.0572 d-1
C) 3.12 d-1
D) 0.319 d-1

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


