Exam 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry
Exam 1: The Atom in Modern Chemistry7 Questions
Exam 2: Chemical Formulas, Chemical Equations, and Reaction Yields15 Questions
Exam 3: Introduction to Quantum Mechanics and Chemical Bonding7 Questions
Exam 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure9 Questions
Exam 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules10 Questions
Exam 6: Solutions12 Questions
Exam 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium12 Questions
Exam 8: Chemical Equilibrium13 Questions
Exam 9: Acidûbase Equilibria11 Questions
Exam 10: Solubility and Precipitation Equilibria6 Questions
Exam 11: Electrochemistry12 Questions
Exam 12: Chemical Kinetics12 Questions
Exam 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry11 Questions
Select questions type
Which of the following is not an acceptable location for an atom in a side-centered primitive cell?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
D
Which of the following will have the maximum ultraviolet absorbance at the longest wavelength?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample initially?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
An element crystallizes in a body-centered cubic lattice, with a = 4.109 Å. If the element has a density of 9.95 g·cm-3, what is the molar mass of the element?
(Multiple Choice)
4.9/5  (40)
(40)
Which estimates the number of electrons per cubic centimeter excited to the conduction band as a function of temperature and the band gap energy. 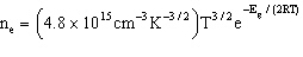 -Refer to Exhibit 22-2. The band gap of pure crystalline silicon is 1.94´10-19 J at 300 K. How many electrons are excited from the valence band to the conduction band at this temperature in a 1.00 cm3 silicon crystal?
-Refer to Exhibit 22-2. The band gap of pure crystalline silicon is 1.94´10-19 J at 300 K. How many electrons are excited from the valence band to the conduction band at this temperature in a 1.00 cm3 silicon crystal?
(Multiple Choice)
4.7/5  (34)
(34)
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. How long will it take for the activity of the 0.25 g sample to fall below 1 Ci? (1 Ci=3.7´1010 Bq)
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following symbols also represents a b particle?
(Multiple Choice)
4.8/5  (39)
(39)
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the activity of the 0.25 gram sample after 30 days?
(Multiple Choice)
4.8/5  (39)
(39)
pertain to a 450 gram sample of 60Co recovered from a decommissioned nuclear reactor. An activity of 1.11´1013 Bq was measured by technicians dismantling the plant.
-Refer to Exhibit 19-2. The activity of the sample was measured a year later and found to be 9.73´1012 Bq. What is the decay constant?
(Multiple Choice)
4.8/5  (46)
(46)
pertain to a 0.25 gram sample of the 32P nuclide which has a half life of 14.28 days and a specific activity of 1.07´1016 Bq·g-1.
-Refer to Exhibit 19-1. What is the value of the decay constant k?
(Multiple Choice)
4.8/5  (33)
(33)
Which estimates the number of electrons per cubic centimeter excited to the conduction band as a function of temperature and the band gap energy. 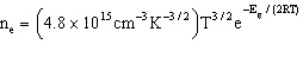 -Refer to Exhibit 22-2. Based solely on the equation above, which set of conditions will result in the most electrons being excited to the conduction band.
-Refer to Exhibit 22-2. Based solely on the equation above, which set of conditions will result in the most electrons being excited to the conduction band.
(Multiple Choice)
4.7/5  (31)
(31)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)