Deck 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules
1
At 20°C water has a vapor pressure of 0.0231 atm. If you bubble air through a container of water and collect 1 L of it at a total pressure of 1 atm, how many grams of water are in your sample of air (you can assume it is saturated)?
A) 9.6´10-4
B) 3.0´10-4
C) 3.0´10-3
D) 1.1´10-2
E) 1.7´10-2
A) 9.6´10-4
B) 3.0´10-4
C) 3.0´10-3
D) 1.1´10-2
E) 1.7´10-2
1.7´10-2
2
Arrange the follow in order of increasing boiling point: H2O, H2S, H2Se, H2Te
A) H2O, H2S, H2Se, H2Te
B) H2Te, H2Se, H2S, H2O
C) H2O, H2Te, H2Se, H2S
D) H2S, H2Se, H2Te, H2O
E) H2S, H2Se, H2O, H2Te
A) H2O, H2S, H2Se, H2Te
B) H2Te, H2Se, H2S, H2O
C) H2O, H2Te, H2Se, H2S
D) H2S, H2Se, H2Te, H2O
E) H2S, H2Se, H2O, H2Te
H2S, H2Se, H2Te, H2O
3
A gas has a temperature of 34.9°C and a volume of 2.8L. It is cooled at constant pressure and the new volume is 2.5 L. What is the new temperature of the gas?
A) 1.89°C
B) 11.6°C
C) 25.2°C
D) 31.2°C
E) 39.1°C
A) 1.89°C
B) 11.6°C
C) 25.2°C
D) 31.2°C
E) 39.1°C
1.89°C
4
The vibration frequency of HCl is 8.66´1013 s-1 and HBr is 7.68´1013 s-1. At 1000 K which molecules will have a larger population in the first excited vibrational state?
A) HCl
B) HBr
C) they will be exactly equal
D) it depends on the force constant of the bond
E) it depends on the total pressure
A) HCl
B) HBr
C) they will be exactly equal
D) it depends on the force constant of the bond
E) it depends on the total pressure

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Helium effuses through a small opening at a rate of 1´10-9 mol s-1. An unknown gas at the same temperature and pressure is found to effuse through the same opening at a rate of 3.78´10-10 mol s-1. What is the molecular mass of the unknown gas?
A) 4 g mol-1
B) 14 g mol-1
C) 18 g mol-1
D) 28 g mol-1
E) 40 g mol-1
A) 4 g mol-1
B) 14 g mol-1
C) 18 g mol-1
D) 28 g mol-1
E) 40 g mol-1

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
Based on the van der Waals equation of state
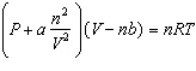
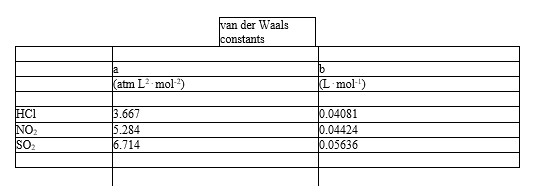
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
A) HCl
B) NO2
C) SO2
D) Must know n to answer this question
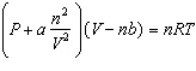
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.

A) HCl
B) NO2
C) SO2
D) Must know n to answer this question

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
With respect to the Maxwell-Boltzmann probability distribution function of molecular speeds, which of the following is true?
A)
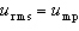
B)
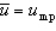
C)
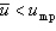
D)
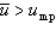
E) A and D
A)
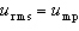
B)
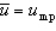
C)
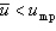
D)
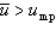
E) A and D

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Name the following compound. 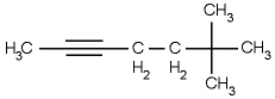
A) 6,6,6-trimethyl-2-hexyne
B) 5,5,5-trimethyl-2-hexyne
C) 6,6-dimethyl-2-heptyne
D) 1,5,5,5-tetrametyl-1-pentyne
E) 5,5-dimethyl-2-hexyne

A) 6,6,6-trimethyl-2-hexyne
B) 5,5,5-trimethyl-2-hexyne
C) 6,6-dimethyl-2-heptyne
D) 1,5,5,5-tetrametyl-1-pentyne
E) 5,5-dimethyl-2-hexyne

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following molecules is chiral
A)
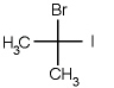
B)
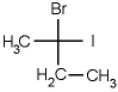
C)
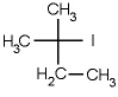
D)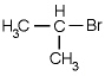
E) none of the above
A)

B)

C)

D)

E) none of the above

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a primary amines?
A)
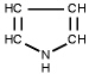
B)
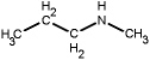
C)
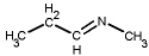
D)
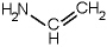
E)
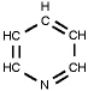
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


