Exam 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules
Exam 1: The Atom in Modern Chemistry7 Questions
Exam 2: Chemical Formulas, Chemical Equations, and Reaction Yields15 Questions
Exam 3: Introduction to Quantum Mechanics and Chemical Bonding7 Questions
Exam 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure9 Questions
Exam 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules10 Questions
Exam 6: Solutions12 Questions
Exam 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium12 Questions
Exam 8: Chemical Equilibrium13 Questions
Exam 9: Acidûbase Equilibria11 Questions
Exam 10: Solubility and Precipitation Equilibria6 Questions
Exam 11: Electrochemistry12 Questions
Exam 12: Chemical Kinetics12 Questions
Exam 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry11 Questions
Select questions type
At 20°C water has a vapor pressure of 0.0231 atm. If you bubble air through a container of water and collect 1 L of it at a total pressure of 1 atm, how many grams of water are in your sample of air (you can assume it is saturated)?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
E
Which of the following is a primary amines?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
With respect to the Maxwell-Boltzmann probability distribution function of molecular speeds, which of the following is true?
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
D
Arrange the follow in order of increasing boiling point: H2O, H2S, H2Se, H2Te
(Multiple Choice)
4.8/5  (34)
(34)
A gas has a temperature of 34.9°C and a volume of 2.8L. It is cooled at constant pressure and the new volume is 2.5 L. What is the new temperature of the gas?
(Multiple Choice)
4.8/5  (45)
(45)
Based on the van der Waals equation of state
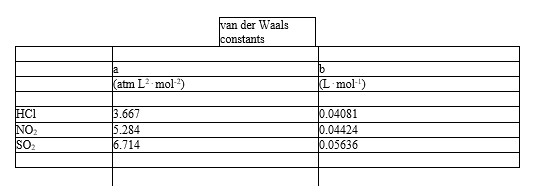
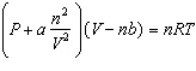 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
(Multiple Choice)
4.9/5  (39)
(39)
The vibration frequency of HCl is 8.66´1013 s-1 and HBr is 7.68´1013 s-1. At 1000 K which molecules will have a larger population in the first excited vibrational state?
(Multiple Choice)
4.9/5  (31)
(31)
Helium effuses through a small opening at a rate of 1´10-9 mol s-1. An unknown gas at the same temperature and pressure is found to effuse through the same opening at a rate of 3.78´10-10 mol s-1. What is the molecular mass of the unknown gas?
(Multiple Choice)
4.8/5  (48)
(48)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)