Deck 3: Introduction to Quantum Mechanics and Chemical Bonding
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/7
Play
Full screen (f)
Deck 3: Introduction to Quantum Mechanics and Chemical Bonding
1
The position of an electron in a Be2+ ion is known to 5.0´10-11 m. What is the uncertainty in its velocity?
A) 2.0´105 m s-1
B) 1.2´106 m s-1
C) 4.6´106 m s-1
D) 9.2´106 m s-1
E) 3.0´108 m s-1
A) 2.0´105 m s-1
B) 1.2´106 m s-1
C) 4.6´106 m s-1
D) 9.2´106 m s-1
E) 3.0´108 m s-1
1.2´106 m s-1
2
If the bond in carbon monoxide is modeled as a harmonic oscillator the force constant is 1860 N m-1, and the reduced mass is 6.86 amu. What is the energy of the ground vibrational state of CO?
A) 1.35´10-21 J
B) 2.71´10-21 J
C) 3.53´10-21 J
D) 2.13´10-20 J
E) 4.26´10-20 J
A) 1.35´10-21 J
B) 2.71´10-21 J
C) 3.53´10-21 J
D) 2.13´10-20 J
E) 4.26´10-20 J
2.13´10-20 J
3
The work function for aluminum is 6.53´10-19 J. If a surface of aluminum is irradiated with 400 nm what is the maximum kinetic energy of the electrons emitted from the surface due to the photo-electric effect?
A) 4.13´105 m s-1
B) 5.85´105 m s-1
C) 1.04´106 m s-1
D) 1.20´106 m s-1
E) no electrons will be emitted
A) 4.13´105 m s-1
B) 5.85´105 m s-1
C) 1.04´106 m s-1
D) 1.20´106 m s-1
E) no electrons will be emitted
no electrons will be emitted
4
Proper wavefunctions ( 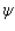 ) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
A)
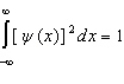
B)
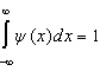
C)
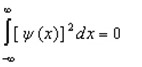
D)
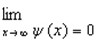
E)
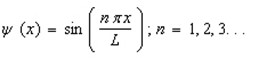
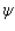 ) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?A)
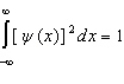
B)
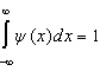
C)
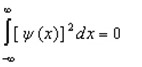
D)
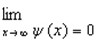
E)
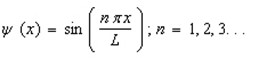

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
5
Before the discovery of the element germanium in 1886, its existence was predicted by Mendeleev as "eka-silicon". Mendeleev not only predicted the existence of this element but also its properties. Use the density of the neighboring elements to estimate the density of Ge. 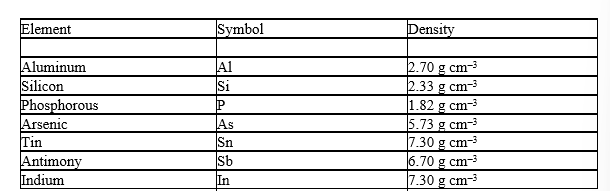
A) 2.5 g cm-3
B) 4 g cm-3
C) 5.5 g cm-3
D) 7 g cm-3
E) 9.6 g cm-3

A) 2.5 g cm-3
B) 4 g cm-3
C) 5.5 g cm-3
D) 7 g cm-3
E) 9.6 g cm-3

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
6
Given the following the data, what is the energy change for this reaction?  Na(g) + Cl(g) Na+(g) + Cl-(g)
Na(g) + Cl(g) Na+(g) + Cl-(g)
A) -1198.4 kJ mol-1
B) -146.8 kJ mol-1
C) +146.8 kJ mol-1
D) +672.6 kJ mol-1
E) +1198.4 kJ mol-1
 Na(g) + Cl(g) Na+(g) + Cl-(g)
Na(g) + Cl(g) Na+(g) + Cl-(g)A) -1198.4 kJ mol-1
B) -146.8 kJ mol-1
C) +146.8 kJ mol-1
D) +672.6 kJ mol-1
E) +1198.4 kJ mol-1

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the best Lewis diagram for formaldehyde, CH2O?
A)
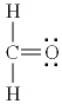
B)
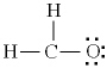
C)
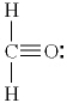
D)
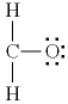
E)
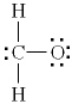
A)

B)
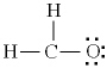
C)

D)
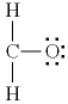
E)


Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck


