Exam 3: Introduction to Quantum Mechanics and Chemical Bonding
Exam 1: The Atom in Modern Chemistry7 Questions
Exam 2: Chemical Formulas, Chemical Equations, and Reaction Yields15 Questions
Exam 3: Introduction to Quantum Mechanics and Chemical Bonding7 Questions
Exam 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure9 Questions
Exam 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules10 Questions
Exam 6: Solutions12 Questions
Exam 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium12 Questions
Exam 8: Chemical Equilibrium13 Questions
Exam 9: Acidûbase Equilibria11 Questions
Exam 10: Solubility and Precipitation Equilibria6 Questions
Exam 11: Electrochemistry12 Questions
Exam 12: Chemical Kinetics12 Questions
Exam 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry11 Questions
Select questions type
Before the discovery of the element germanium in 1886, its existence was predicted by Mendeleev as "eka-silicon". Mendeleev not only predicted the existence of this element but also its properties. Use the density of the neighboring elements to estimate the density of Ge. 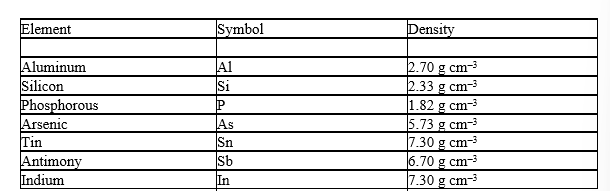
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
C
Proper wavefunctions ( 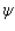 ) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
) in quantum mechanics must be normalized. Which statement below best summarizes this mathematically?
Free
(Multiple Choice)
4.9/5  (49)
(49)
Correct Answer:
A
The position of an electron in a Be2+ ion is known to 5.0´10-11 m. What is the uncertainty in its velocity?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
B
The work function for aluminum is 6.53´10-19 J. If a surface of aluminum is irradiated with 400 nm what is the maximum kinetic energy of the electrons emitted from the surface due to the photo-electric effect?
(Multiple Choice)
4.9/5  (39)
(39)
If the bond in carbon monoxide is modeled as a harmonic oscillator the force constant is 1860 N m-1, and the reduced mass is 6.86 amu. What is the energy of the ground vibrational state of CO?
(Multiple Choice)
4.9/5  (41)
(41)
Given the following the data, what is the energy change for this reaction?  Na(g) + Cl(g) Na+(g) + Cl-(g)
Na(g) + Cl(g) Na+(g) + Cl-(g)
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is the best Lewis diagram for formaldehyde, CH2O?
(Multiple Choice)
4.8/5  (26)
(26)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)