Deck 3: Oxygen Transporters: Hemoglobin and Myoglobin
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 3: Oxygen Transporters: Hemoglobin and Myoglobin
1
Fetal hemoglobin has a somewhat higher oxygen-binding affinity than does adult hemoglobin because:
A)It has only one subunit, and therefore there is no cooperativity of oxygen binding.
B)2,3-BPG binds less tightly to fetal hemoglobin than to adult hemoglobin, and this weakens its effect on the oxygen-binding affinity.
C)The noncovalent interactions between the subunits are stronger in fetal hemoglobin than in adult hemoglobin.
D)Fetal hemoglobin is less acidic than adult hemoglobin, and therefore it is less affected by increased acidity in the erythrocyte.
E)The terminal amino groups of fetal hemoglobin are chemically modified, and therefore they cannot react with carbon dioxide.
A)It has only one subunit, and therefore there is no cooperativity of oxygen binding.
B)2,3-BPG binds less tightly to fetal hemoglobin than to adult hemoglobin, and this weakens its effect on the oxygen-binding affinity.
C)The noncovalent interactions between the subunits are stronger in fetal hemoglobin than in adult hemoglobin.
D)Fetal hemoglobin is less acidic than adult hemoglobin, and therefore it is less affected by increased acidity in the erythrocyte.
E)The terminal amino groups of fetal hemoglobin are chemically modified, and therefore they cannot react with carbon dioxide.
2,3-BPG binds less tightly to fetal hemoglobin than to adult hemoglobin, and this weakens its effect on the oxygen-binding affinity.
2
The quaternary structure of deoxyhemoglobin is maintained, in part, by which type of bond?
A)Heme-to-heme hydrophobic interactions.
B)Disulfide bonds.
C)Metallic coordination bonds.
D)Hydrogen bonds formed by covalently bound carbohydrate groups.
E)Salt bonds.
A)Heme-to-heme hydrophobic interactions.
B)Disulfide bonds.
C)Metallic coordination bonds.
D)Hydrogen bonds formed by covalently bound carbohydrate groups.
E)Salt bonds.
Salt bonds.
3
The effect of high 2,3-BPG in red blood cells is:
A)To maintain the reducing environment and prevent methemoglobin formation.
B)To facilitate tetramer formation from α and β subunit monomers.
C)To increase the P50 of hemoglobin.
D)To convert the oxygen saturation curve of hemoglobin from sigmoid to hyperbolic.
E)To increase the rate of conversion of the T to the R form.
A)To maintain the reducing environment and prevent methemoglobin formation.
B)To facilitate tetramer formation from α and β subunit monomers.
C)To increase the P50 of hemoglobin.
D)To convert the oxygen saturation curve of hemoglobin from sigmoid to hyperbolic.
E)To increase the rate of conversion of the T to the R form.
To increase the P50 of hemoglobin.
4
An experienced hiker, H.A., spends her vacation in Tibet.She complains of often feeling dizzy at high altitudes.Which of the following changes in the cytosol of H.A.'s red blood cells will result in the delivery of more oxygen to her tissues at the relatively low pO₂ on Mount Everest?
A)A decrease in methemoglobin reductase activity.
B)A higher rate of γ-globin gene transcription in red blood cells.
C)An increased blood pH.
D)An increase in the 2,3-BPG concentration.
E)An increase in the carbon dioxide partial pressure.
A)A decrease in methemoglobin reductase activity.
B)A higher rate of γ-globin gene transcription in red blood cells.
C)An increased blood pH.
D)An increase in the 2,3-BPG concentration.
E)An increase in the carbon dioxide partial pressure.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
A solution of equal amounts of hemoglobin and myoglobin is maintained at a very low partial pressure of oxygen, so that both proteins exist predominantly in the deoxygenated form.What happens when the solution is then bubbled with a small amount of oxygen?
A)Myoglobin undergoes a conformational change that raises its oxygen affinity above that of hemoglobin.
B)2,3-BPG dissociates from hemoglobin and binds to myoglobin.
C)Much more of the dissolved oxygen binds to myoglobin than to hemoglobin.
D)Hemoglobin dissociates into its subunits.
E)Most hemoglobin becomes oxygenated, whereas most myoglobin remains deoxygenated.
A)Myoglobin undergoes a conformational change that raises its oxygen affinity above that of hemoglobin.
B)2,3-BPG dissociates from hemoglobin and binds to myoglobin.
C)Much more of the dissolved oxygen binds to myoglobin than to hemoglobin.
D)Hemoglobin dissociates into its subunits.
E)Most hemoglobin becomes oxygenated, whereas most myoglobin remains deoxygenated.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
In Figure 3.2, the solid line depicts normal hemoglobin. 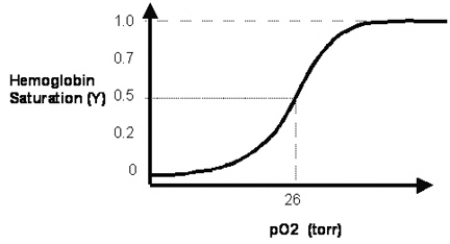 How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?
How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?
A)The oxygen half-saturation pressure value P50 of the new hemoglobin saturation curve is reduced.
B)The maximal saturation of hemoglobin with oxygen is reduced to less than a value of 1.0.
C)The oxygen partial pressure (pO₂) at which hemoglobin attains a Y-axis value of 0.25 is increased.
D)The new curve has a Y-axis value of 0.25 at a pO₂ of 26 mm Hg.
E)The new curve has a reduced slope at the P50 value of 26 mm Hg.
 How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?
How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?A)The oxygen half-saturation pressure value P50 of the new hemoglobin saturation curve is reduced.
B)The maximal saturation of hemoglobin with oxygen is reduced to less than a value of 1.0.
C)The oxygen partial pressure (pO₂) at which hemoglobin attains a Y-axis value of 0.25 is increased.
D)The new curve has a Y-axis value of 0.25 at a pO₂ of 26 mm Hg.
E)The new curve has a reduced slope at the P50 value of 26 mm Hg.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about hemoglobin is correct?
A)Heme is the cosubstrate for hemoglobin.
B)There are six coordinate bonds formed by Fe+2 in deoxyhemoglobin.
C)Methemoglobin is the functional oxygenated form of hemoglobin.
D)Hemoglobin binds oxygen with lower affinity than myoglobin.
E)Hemoglobin is a tetrameric protein with four identical polypeptide chains.
A)Heme is the cosubstrate for hemoglobin.
B)There are six coordinate bonds formed by Fe+2 in deoxyhemoglobin.
C)Methemoglobin is the functional oxygenated form of hemoglobin.
D)Hemoglobin binds oxygen with lower affinity than myoglobin.
E)Hemoglobin is a tetrameric protein with four identical polypeptide chains.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
The oxygen dissociation curve of hemoglobin is sigmoidal, and this raises the efficiency of oxygen delivery considerably.The reason for hemoglobin's sigmoidal oxygen dissociation curve is:
A)The steric control of oxygen access to the heme iron by the distal histidine.
B)The difference in oxygen affinity between the heme groups of the α chains and those of the β chains.
C)A conformational change in the protein that raises the oxygen-binding affinities of the other heme groups when one of them becomes oxygenated.
D)The effect of 2,3-BG, which binds only to oxygenated but not deoxygenated hemoglobin.
E)The hydrophobicity of the heme-binding pocket, which forces oxygen to diffuse through a hydrophobic medium in order to reach the heme iron.
A)The steric control of oxygen access to the heme iron by the distal histidine.
B)The difference in oxygen affinity between the heme groups of the α chains and those of the β chains.
C)A conformational change in the protein that raises the oxygen-binding affinities of the other heme groups when one of them becomes oxygenated.
D)The effect of 2,3-BG, which binds only to oxygenated but not deoxygenated hemoglobin.
E)The hydrophobicity of the heme-binding pocket, which forces oxygen to diffuse through a hydrophobic medium in order to reach the heme iron.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
A 26-year-old scuba diver returns after a brief dive, complaining of breathlessness, dizziness, and nausea.The dive shop owner quickly realizes that his assistant has equipped the diver with a CO₂ breathing tank, instead of an O₂ breathing tank.How would the rapid increase in pCO₂ concentration in the diver's lungs change the oxygen saturation curve of hemoglobin shown in Figure 3.4? 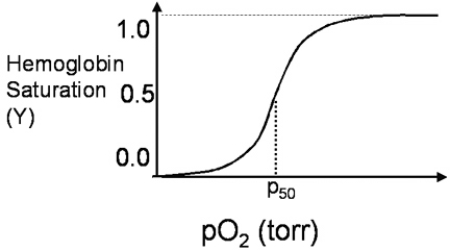
A)It shifts the maximal saturation value from 1.0 to 0.8.
B)It increases the P50 value of hemoglobin.
C)It changes the curve to a hyperbolic shape with the same P50.
D)It makes the slope of the curve steeper at the P50.
E)It has no effect on the curve shown.

A)It shifts the maximal saturation value from 1.0 to 0.8.
B)It increases the P50 value of hemoglobin.
C)It changes the curve to a hyperbolic shape with the same P50.
D)It makes the slope of the curve steeper at the P50.
E)It has no effect on the curve shown.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Most of the carbon dioxide that is formed during oxidative metabolism is transported in the blood:
A)Covalently bound to the C-terminal amino acids of the hemoglobin chains.
B)Covalently bound to the N-terminal amino acids of the hemoglobin chains.
C)In the form of inorganic bicarbonate ion (HCO₃-).
D)In the form of carbonic acid (H₂CO₃).
E)And physically dissolves as CO₂ in the blood.
A)Covalently bound to the C-terminal amino acids of the hemoglobin chains.
B)Covalently bound to the N-terminal amino acids of the hemoglobin chains.
C)In the form of inorganic bicarbonate ion (HCO₃-).
D)In the form of carbonic acid (H₂CO₃).
E)And physically dissolves as CO₂ in the blood.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


