Exam 3: Oxygen Transporters: Hemoglobin and Myoglobin
Exam 1: Introduction to Biomolecules4 Questions
Exam 2: Introduction to Protein Structure12 Questions
Exam 3: Oxygen Transporters: Hemoglobin and Myoglobin10 Questions
Exam 4: Enzymatic Reactions20 Questions
Exam 5: Coenzymes4 Questions
Exam 6: DNA, RNA, and Protein Synthesis25 Questions
Exam 7: The Human Genome14 Questions
Exam 8: Protein Targeting4 Questions
Exam 9: Introduction to Genetic Diseases10 Questions
Exam 10: Viruses4 Questions
Exam 11: DNA Technology15 Questions
Exam 12: Biological Membranes6 Questions
Exam 13: The Cytoskeleton6 Questions
Exam 14: The Extracellular Matrix7 Questions
Exam 15: Plasma Proteins19 Questions
Exam 16: Extracellular Messengers11 Questions
Exam 17: Intracellular Messengers12 Questions
Exam 18: Cellular Growth Control and Cancer12 Questions
Exam 19: Digestive Enzymes3 Questions
Exam 20: Introduction to Metabolic Pathways2 Questions
Exam 21: Glycolysis, Tricarboxylic Acid Cycle, and Oxidative Phosphorylation9 Questions
Exam 22: Carbohydrate Metabolism9 Questions
Exam 23: The Metabolism of Fatty Acids and Triglycerides12 Questions
Exam 24: The Metabolism of Membrane Lipids4 Questions
Exam 25: Lipid Transport9 Questions
Exam 26: Amino Acid Metabolism10 Questions
Exam 27: Heme Metabolism3 Questions
Exam 28: The Metabolism of Purines and Pyrimidines8 Questions
Exam 29: Vitamins and Minerals16 Questions
Exam 30: Integration of Metabolism15 Questions
Select questions type
The quaternary structure of deoxyhemoglobin is maintained, in part, by which type of bond?
Free
(Multiple Choice)
5.0/5  (34)
(34)
Correct Answer:
E
A solution of equal amounts of hemoglobin and myoglobin is maintained at a very low partial pressure of oxygen, so that both proteins exist predominantly in the deoxygenated form.What happens when the solution is then bubbled with a small amount of oxygen?
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
C
The effect of high 2,3-BPG in red blood cells is:
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
C
An experienced hiker, H.A., spends her vacation in Tibet.She complains of often feeling dizzy at high altitudes.Which of the following changes in the cytosol of H.A.'s red blood cells will result in the delivery of more oxygen to her tissues at the relatively low pO₂ on Mount Everest?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following statements about hemoglobin is correct?
(Multiple Choice)
4.9/5  (43)
(43)
Most of the carbon dioxide that is formed during oxidative metabolism is transported in the blood:
(Multiple Choice)
5.0/5  (36)
(36)
In Figure 3.2, the solid line depicts normal hemoglobin. 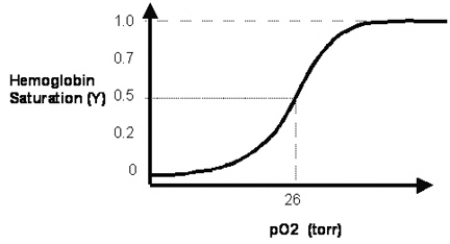 How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?
How does the normal curve change when the 2,3-bisphosphoglycerate (2,3-BPG) concentration is reduced?
(Multiple Choice)
4.8/5  (42)
(42)
The oxygen dissociation curve of hemoglobin is sigmoidal, and this raises the efficiency of oxygen delivery considerably.The reason for hemoglobin's sigmoidal oxygen dissociation curve is:
(Multiple Choice)
5.0/5  (31)
(31)
Fetal hemoglobin has a somewhat higher oxygen-binding affinity than does adult hemoglobin because:
(Multiple Choice)
4.8/5  (21)
(21)
A 26-year-old scuba diver returns after a brief dive, complaining of breathlessness, dizziness, and nausea.The dive shop owner quickly realizes that his assistant has equipped the diver with a CO₂ breathing tank, instead of an O₂ breathing tank.How would the rapid increase in pCO₂ concentration in the diver's lungs change the oxygen saturation curve of hemoglobin shown in Figure 3.4? 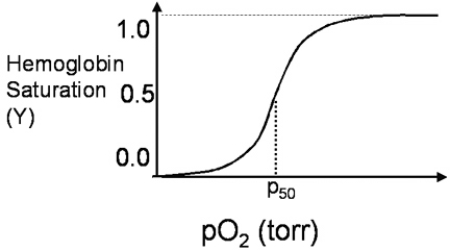
(Multiple Choice)
4.8/5  (37)
(37)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)