Deck 5: Atomic Theory: The Nuclear Model of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 5: Atomic Theory: The Nuclear Model of the Atom
1
Which of the following statements about isotopes is/are correct?
i.They have different masses
ii.They have different numbers of neutrons
iii.They have different electrical charges
iv.They have different nuclear symbols
v.They have different mass numbers
A)i and ii
B)i, ii, and iv
C)i, ii, and v
D)i, ii, iv, and v
E)All are correct
i.They have different masses
ii.They have different numbers of neutrons
iii.They have different electrical charges
iv.They have different nuclear symbols
v.They have different mass numbers
A)i and ii
B)i, ii, and iv
C)i, ii, and v
D)i, ii, iv, and v
E)All are correct
i, ii, iv, and v
2
Which of the following is not one of the main features of Dalton's atomic theory?
A)Each element is made up of tiny, individual particles called atoms
B)Atoms are indivisible; they cannot be created or destroyed
C)Mass differences among atoms of an element are caused by different numbers of neutrons
D)Atoms of one element are different from atoms of any other element
E)Atoms of one element may combine with atoms of another element to form chemical compounds
A)Each element is made up of tiny, individual particles called atoms
B)Atoms are indivisible; they cannot be created or destroyed
C)Mass differences among atoms of an element are caused by different numbers of neutrons
D)Atoms of one element are different from atoms of any other element
E)Atoms of one element may combine with atoms of another element to form chemical compounds
Mass differences among atoms of an element are caused by different numbers of neutrons
3
Which of the following statements is true?
A)Given the atomic number of an element, the atomic mass of the element can be calculated
B)An isotope is identified by its mass number
C)The number of neutrons in an atom determines the chemical reactivity of that isotope
D)The total contribution to the mass of an atom from protons and electrons varies among the atoms of an element
E)Mass differences among atoms of an element cannot be accounted for with our current understanding of the atom
A)Given the atomic number of an element, the atomic mass of the element can be calculated
B)An isotope is identified by its mass number
C)The number of neutrons in an atom determines the chemical reactivity of that isotope
D)The total contribution to the mass of an atom from protons and electrons varies among the atoms of an element
E)Mass differences among atoms of an element cannot be accounted for with our current understanding of the atom
An isotope is identified by its mass number
4
Consider the following model of a generic atom. 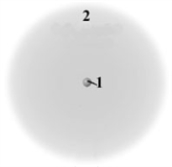 Which of the following is correct regarding the "regions" labeled 1 and 2?
Which of the following is correct regarding the "regions" labeled 1 and 2?
A)Region 1 contains most of the mass and is negatively charged.
B)Region 2 contains very little mass and is positively charged.
C)Region 1 contains most of the mass and is positively charged.
D)Region 2 contains very little mass and is not charged.
E)Region 1 contains most of the mass and not charged.
 Which of the following is correct regarding the "regions" labeled 1 and 2?
Which of the following is correct regarding the "regions" labeled 1 and 2?A)Region 1 contains most of the mass and is negatively charged.
B)Region 2 contains very little mass and is positively charged.
C)Region 1 contains most of the mass and is positively charged.
D)Region 2 contains very little mass and is not charged.
E)Region 1 contains most of the mass and not charged.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is not a conclusion that follows from the results of the Rutherford scattering experiment?
A)Every atom contains an extremely small, extremely dense nucleus
B)All of the positive charge and nearly all of the mass of an atom are concentrated in the nucleus
C)The nucleus is surrounded by a much larger volume of empty space that makes up the rest of the atom
D)When two elements combine to form more than one compound, the different weights of one element that combine with the same weight of the other element are in a simple ratio of whole numbers
E)The space outside the nucleus is very thinly populated by electrons
A)Every atom contains an extremely small, extremely dense nucleus
B)All of the positive charge and nearly all of the mass of an atom are concentrated in the nucleus
C)The nucleus is surrounded by a much larger volume of empty space that makes up the rest of the atom
D)When two elements combine to form more than one compound, the different weights of one element that combine with the same weight of the other element are in a simple ratio of whole numbers
E)The space outside the nucleus is very thinly populated by electrons

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an incorrect statement about the Rutherford scattering experiment?
A)Most of the alpha particles directed at a very thin gold foil passed right through the foil, undeflected from their straight-line path
B)As a result of the experiment, the atom is pictured as consisting mostly of open space
C)The few alpha particles that were on a collision course with the gold electrons were repelled backward at acute angles
D)The experiment led to the conclusion that the nucleus of an atom contains all of the atom's positive charge
E)The experiment provided a partial understanding of the location and charge of the particles within the atom
A)Most of the alpha particles directed at a very thin gold foil passed right through the foil, undeflected from their straight-line path
B)As a result of the experiment, the atom is pictured as consisting mostly of open space
C)The few alpha particles that were on a collision course with the gold electrons were repelled backward at acute angles
D)The experiment led to the conclusion that the nucleus of an atom contains all of the atom's positive charge
E)The experiment provided a partial understanding of the location and charge of the particles within the atom

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a correct statement about the Rutherford scattering experiment?
A)The experiment provided an explanation of the discrete lines in atomic spectra
B)The experiment yielded evidence that electrons are charged particles but neutrons are not electrically charged
C)The experiment showed that the electrons contain nearly all of the mass of the atom
D)The experiment provided evidence that matter does not consist of atoms
E)The experiment indicated that at the center of the atom is a tiny and extremely dense nucleus
A)The experiment provided an explanation of the discrete lines in atomic spectra
B)The experiment yielded evidence that electrons are charged particles but neutrons are not electrically charged
C)The experiment showed that the electrons contain nearly all of the mass of the atom
D)The experiment provided evidence that matter does not consist of atoms
E)The experiment indicated that at the center of the atom is a tiny and extremely dense nucleus

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
The chemical symbol of germanium is Ge.An isotope of this element has 32 protons and 46 neutrons in the nucleus.What are the nuclear symbol,the atomic number,and the mass number of this isotope of germanium?
Nuclear symbol Atomic number Mass number
A)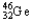 46 32
46 32
B)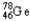 78 46
78 46
C)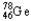 46 78
46 78
D)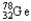 78 32
78 32
E)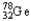 32 78
32 78
Nuclear symbol Atomic number Mass number
A)
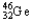 46 32
46 32B)
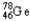 78 46
78 46C)
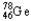 46 78
46 78D)
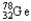 78 32
78 32E)
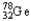 32 78
32 78
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is false?
A)The proton and the neutron are primarily responsible for the mass of an atom
B)The charge on electrons and protons are equal in magnitude but opposite in sign
C)The mass of a proton and a neutron are approximately the same
D)The mass of a proton is approximately one gram
E)A neutron, as its name suggests, is electrically neutral
A)The proton and the neutron are primarily responsible for the mass of an atom
B)The charge on electrons and protons are equal in magnitude but opposite in sign
C)The mass of a proton and a neutron are approximately the same
D)The mass of a proton is approximately one gram
E)A neutron, as its name suggests, is electrically neutral

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
The nuclear symbol of an isotope of beryllium is 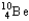 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.

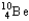 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
Dalton's atomic theory explained the observation that the percentage by mass of the elements in a compound is always the same,thus Dalton's atomic theory supports what Law?
A)The Law of Definite Composition
B)The Law of Conservation of Mass
C)The Law of Conservation of Energy
D)The Law of Multiple Proportions
E)The Law of Chemical Compounds
A)The Law of Definite Composition
B)The Law of Conservation of Mass
C)The Law of Conservation of Energy
D)The Law of Multiple Proportions
E)The Law of Chemical Compounds

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
Isotopes of an element vary in mass because of differing numbers of:
A)Protons
B)Neutrons
C)Electrons
D)Protons and neutrons
E)Protons and electrons
A)Protons
B)Neutrons
C)Electrons
D)Protons and neutrons
E)Protons and electrons

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is false?
A)Atoms of the same element that have different masses are called isotopes
B)The masses of isotopes vary because they have different numbers of electrons
C)The mass number of an isotope is the total number of protons and neutrons in the nucleus
D)You can find the mass number of an atom given the number of protons and neutrons, but without being given the number of electrons
E)Every atom of a particular element has the same number of protons
A)Atoms of the same element that have different masses are called isotopes
B)The masses of isotopes vary because they have different numbers of electrons
C)The mass number of an isotope is the total number of protons and neutrons in the nucleus
D)You can find the mass number of an atom given the number of protons and neutrons, but without being given the number of electrons
E)Every atom of a particular element has the same number of protons

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
Dalton's atomic theory led to a prediction that must be true if the theory is correct: when two elements combine to form a compound,the masses in which they combine is constant..What is the name of this Law that followed from Dalton's atomic theory?
A)The Law of Weight Ratios
B)The Law of Multiple Proportions
C)The Law of Definite Composition
D)The Law of Conservation of Weight
E)The Law of Conservation of Mass
A)The Law of Weight Ratios
B)The Law of Multiple Proportions
C)The Law of Definite Composition
D)The Law of Conservation of Weight
E)The Law of Conservation of Mass

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the three major subatomic particles has/have a mass of approximately 1 u?
i.e-
ii.p+
iii.n0
A)i only
B)ii only
C)iii only
D)i and ii
E)ii and iii
i.e-
ii.p+
iii.n0
A)i only
B)ii only
C)iii only
D)i and ii
E)ii and iii

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the following generic picture of an atom. 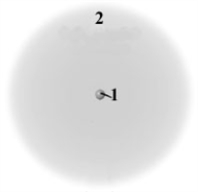 Which of the following is correct about the regions labeled 1 and 2?
Which of the following is correct about the regions labeled 1 and 2?
A)Region 2 is populated by electrons and neutrons.
B)Region 2 is populated by electrons an protons.
C)Region 1 is populated by protons and neutrons.
D)Region 1 is populated by protons and electrons.
E)Region 1 is populated only by electrons and Region 2 only by protons.
 Which of the following is correct about the regions labeled 1 and 2?
Which of the following is correct about the regions labeled 1 and 2?A)Region 2 is populated by electrons and neutrons.
B)Region 2 is populated by electrons an protons.
C)Region 1 is populated by protons and neutrons.
D)Region 1 is populated by protons and electrons.
E)Region 1 is populated only by electrons and Region 2 only by protons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the three major subatomic particles has/have a charge of -1?
i.electron
ii.proton
iii.neutron
A)i only
B)ii only
C)iii only
D)i and ii
E)ii and iii
i.electron
ii.proton
iii.neutron
A)i only
B)ii only
C)iii only
D)i and ii
E)ii and iii

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is not a description of atoms according to Dalton's atomic theory?
A)Atoms cannot be destroyed or created
B)Atoms of different elements may combine in the ratio of small, whole numbers to form compounds
C)Atoms of the same element that have different masses are called isotopes
D)All atoms of one element have the same mass
E)Atoms of two different elements have different masses
A)Atoms cannot be destroyed or created
B)Atoms of different elements may combine in the ratio of small, whole numbers to form compounds
C)Atoms of the same element that have different masses are called isotopes
D)All atoms of one element have the same mass
E)Atoms of two different elements have different masses

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the following pictorial representation of the postulates of Dalton's atomic theory. 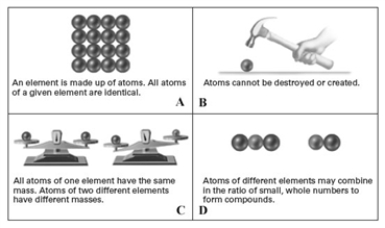 Which of the following is the best illustrates of the law of multiple proportions?
Which of the following is the best illustrates of the law of multiple proportions?
A)A
B)B
C)C
D)D
E)None of these illustrate the law of multiple proportions.
 Which of the following is the best illustrates of the law of multiple proportions?
Which of the following is the best illustrates of the law of multiple proportions?A)A
B)B
C)C
D)D
E)None of these illustrate the law of multiple proportions.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
The nuclear symbol of an isotope of potassium is 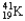 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.

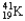 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481 u,22.122% at a mass of 283.99570 u,and the remainder has a mass of 286.99423 u.Calculate the atomic mass of the element.
A)281.99 u
B)283.29 u
C)284.00 u
D)284.33 u
E)286.99 u
A)281.99 u
B)283.29 u
C)284.00 u
D)284.33 u
E)286.99 u

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following elements are correctly matched with their period and group?

A)v only
B)i, ii, iii, and iv
C)ii, iii, and iv
D)i, ii, and iii
E)ii and iii

A)v only
B)i, ii, iii, and iv
C)ii, iii, and iv
D)i, ii, and iii
E)ii and iii

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is/are false?
i.Groups are arranged vertically in the periodic table
ii.Periods are arranged horizontally in the periodic table
iii.Periods are numbered from top to bottom
iv.There are eight elements in the first period of the periodic table
A)i only
B)ii and iii
C)i and ii
D)iv only
E)i, ii, and iv
i.Groups are arranged vertically in the periodic table
ii.Periods are arranged horizontally in the periodic table
iii.Periods are numbered from top to bottom
iv.There are eight elements in the first period of the periodic table
A)i only
B)ii and iii
C)i and ii
D)iv only
E)i, ii, and iv

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
Groups in the periodic table are also known as...
A)periods
B)IUPACs
C)Mendeleevian elements
D)chemical families
E)assemblages
A)periods
B)IUPACs
C)Mendeleevian elements
D)chemical families
E)assemblages

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is the best definition of the atomic mass unit?
A)Exactly 1/12 of the mass of a carbon-12 atom
B)The average mass of the atoms of an element compared to an atom of carbon-12 at exactly 12 u
C)The total number of protons and neutrons in the nucleus of an atom of carbon-12
D)Exactly 1/12 of the mass of a carbon-12 nucleus
E)Exactly 1/12 of the mass of 12 neutrons in a neutral electrical field
A)Exactly 1/12 of the mass of a carbon-12 atom
B)The average mass of the atoms of an element compared to an atom of carbon-12 at exactly 12 u
C)The total number of protons and neutrons in the nucleus of an atom of carbon-12
D)Exactly 1/12 of the mass of a carbon-12 nucleus
E)Exactly 1/12 of the mass of 12 neutrons in a neutral electrical field

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
One of the main features of Dalton's atomic theory no longer considered valid is: All atoms of each element are identical in every respect.Which of the following is the best explanation of why this feature is no longer considered valid?
A)The existence of isotopes
B)Mass is conserved in a chemical change
C)Nearly all of the mass of an atom is concentrated in the nucleus
D)The total charge of the electrons of an atom exactly balances the positive charge of the nucleus
E)The planetary model of the atom has been shown to be incorrect
A)The existence of isotopes
B)Mass is conserved in a chemical change
C)Nearly all of the mass of an atom is concentrated in the nucleus
D)The total charge of the electrons of an atom exactly balances the positive charge of the nucleus
E)The planetary model of the atom has been shown to be incorrect

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
Two natural isotopes exist for silver.51.83% of the atoms have a mass of 106.90509 u,and the remaining fraction has a mass of 108.9047 u.Calculate the atomic mass of silver.
A)52.46 u
B)55.41 u
C)106.90509 u
D)107.87 u
E)108.9047 u
A)52.46 u
B)55.41 u
C)106.90509 u
D)107.87 u
E)108.9047 u

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is correct for the element with atomic number 20?



Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the atomic mass of the element having the following distribution of isotopes:
5.8% 53.9396 u
91.8% 55.9349 u
2.1% 56.9354 u
0.3% 57.9333 u
A)51.3 u
B)55.8 u
C)55.9349 u
D)56.2 u
E)5.58 × 103 u
5.8% 53.9396 u
91.8% 55.9349 u
2.1% 56.9354 u
0.3% 57.9333 u
A)51.3 u
B)55.8 u
C)55.9349 u
D)56.2 u
E)5.58 × 103 u

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following gives the correct number of elements in each of the first six periods in the periodic table?
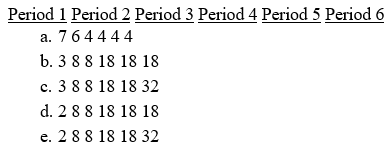


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is false?
A)The atomic mass unit is exactly 1/12 of the mass of a carbon-12 atom
B)Both protons and neutrons have atomic masses very close to 1 u
C)A carbon-12 atom with six electrons has a mass of 12.6 u
D)The atomic mass of an element is the average mass of the atoms of an element compared to an atom of carbon-12 at exactly 12 u
E)To find the atomic mass of an element, you must know the atomic mass of each isotope and the fraction of each isotope in a sample
A)The atomic mass unit is exactly 1/12 of the mass of a carbon-12 atom
B)Both protons and neutrons have atomic masses very close to 1 u
C)A carbon-12 atom with six electrons has a mass of 12.6 u
D)The atomic mass of an element is the average mass of the atoms of an element compared to an atom of carbon-12 at exactly 12 u
E)To find the atomic mass of an element, you must know the atomic mass of each isotope and the fraction of each isotope in a sample

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
The chemical symbol of argon is Ar.An isotope of this element has an atomic number of 18 and a mass number of 38.What are the nuclear symbol,the number of protons,and the number of neutrons of this isotope of argon?
Nuclear symbol Number of protons Number of neutrons
A)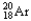 18 38
18 38
B)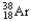 20 18
20 18
C)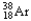 38 18
38 18
D)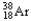 18 38
18 38
E)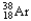 18 20
18 20
Nuclear symbol Number of protons Number of neutrons
A)
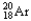 18 38
18 38B)
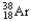 20 18
20 18C)
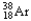 38 18
38 18D)
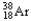 18 38
18 38E)
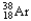 18 20
18 20
Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
Copper has two naturally occurring isotopes with atomic masses of 62.9296 u ( 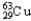 )and 64.9278 u (
)and 64.9278 u ( 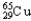 ).The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
).The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
A)69.15%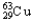 , 30.85%
, 30.85% 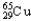
B)30.85%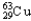 , 69.15%
, 69.15% 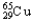
C)1.08%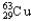 , 98.92%
, 98.92% 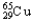
D)98.92%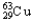 , 1.08%
, 1.08% 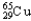
E)99.03%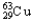 , 0.97%
, 0.97% 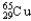
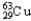 )and 64.9278 u (
)and 64.9278 u ( 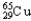 ).The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
).The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?A)69.15%
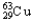 , 30.85%
, 30.85% 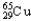
B)30.85%
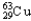 , 69.15%
, 69.15% 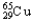
C)1.08%
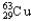 , 98.92%
, 98.92% 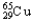
D)98.92%
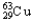 , 1.08%
, 1.08% 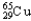
E)99.03%
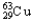 , 0.97%
, 0.97% 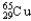

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
One of the main features of Dalton's atomic theory no longer considered valid is: atoms cannot be created or destroyed.Which of the following is the best explanation of why this feature is no longer considered valid?
A)The percentage by mass of the elements in a compound is always the same
B)In a chemical change, mass is neither created or destroyed
C)Every atom contains an extremely small, extremely dense nucleus
D)When two elements combine to form more than one compound, the different weights of one element that combine with the same weight of the other element are in a simple ratio of whole numbers
E)The discovery of subatomic particles
A)The percentage by mass of the elements in a compound is always the same
B)In a chemical change, mass is neither created or destroyed
C)Every atom contains an extremely small, extremely dense nucleus
D)When two elements combine to form more than one compound, the different weights of one element that combine with the same weight of the other element are in a simple ratio of whole numbers
E)The discovery of subatomic particles

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following features of Dalton's atomic theory is/are no longer considered valid?
i.Each element is made up of tiny,individual particles called atoms
ii.Atoms are indivisible; they cannot be created or destroyed
iii.All atoms of each element are identical in every respect
iv.Atoms of one element are different from atoms of any other element
v.Atoms of one element may combine with atoms of another element to form chemical compounds
A)ii only
B)iii only
C)iv only
D)ii and iii
E)ii, iii, and iv
i.Each element is made up of tiny,individual particles called atoms
ii.Atoms are indivisible; they cannot be created or destroyed
iii.All atoms of each element are identical in every respect
iv.Atoms of one element are different from atoms of any other element
v.Atoms of one element may combine with atoms of another element to form chemical compounds
A)ii only
B)iii only
C)iv only
D)ii and iii
E)ii, iii, and iv

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is correct for the element with Z = 9?



Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following masses is closest to the mass of one atomic mass unit?
A)12 g
B)1.66 g
C)1 g
D)1/12 g
E)10-24 g
A)12 g
B)1.66 g
C)1 g
D)1/12 g
E)10-24 g

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is correct?
A)The element H is in both the first period and the seventh period
B)The element Na is in Group 2A/2
C)The element Ge is in the fourth period and Group 4A/14
D)The element Cr is in the third period and Group 6B/6
E)More than one of the statements above are correct
A)The element H is in both the first period and the seventh period
B)The element Na is in Group 2A/2
C)The element Ge is in the fourth period and Group 4A/14
D)The element Cr is in the third period and Group 6B/6
E)More than one of the statements above are correct

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
One of the features of Dalton's atomic theory no longer considered to be correct is: All atoms of each element are identical in every respect.Which of the following is the best explanation of why this feature is no longer considered valid?
A)The existence of isotopes
B)The existence of ions
C)Natural variation in the mass of the proton
D)Natural variation in the mass of the neutron
E)Natural variation in the masses of the proton and the neutron
A)The existence of isotopes
B)The existence of ions
C)Natural variation in the mass of the proton
D)Natural variation in the mass of the neutron
E)Natural variation in the masses of the proton and the neutron

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is the best explanation of why the mass of an atom is usually expressed in atomic mass units rather than grams?
A)An atomic mass unit is only used for carbon-12
B)The mass of one milliliter of water is one gram
C)The mass in grams is not known for atoms
D)The mass of an atom in grams is inconveniently small
E)Metric units such as grams are not applicable to atoms
A)An atomic mass unit is only used for carbon-12
B)The mass of one milliliter of water is one gram
C)The mass in grams is not known for atoms
D)The mass of an atom in grams is inconveniently small
E)Metric units such as grams are not applicable to atoms

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
Examine the block on the periodic table indicated by the arrow. 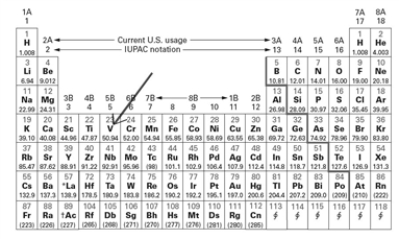 Which of the following correctly describes this element (V - vanadium)?
Which of the following correctly describes this element (V - vanadium)?
A)a metal with 25 neutrons in the nucleus.
B)a transition metal in period 3 group 5.
C)a transition metal in period 4 with 23 protons
D)a nonmetal in period 4 group 5
E)a metal in period 3 group 5 with 23 electrons
 Which of the following correctly describes this element (V - vanadium)?
Which of the following correctly describes this element (V - vanadium)?A)a metal with 25 neutrons in the nucleus.
B)a transition metal in period 3 group 5.
C)a transition metal in period 4 with 23 protons
D)a nonmetal in period 4 group 5
E)a metal in period 3 group 5 with 23 electrons

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is correct for the fourth period element in Group 3A/13?



Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the following figure. 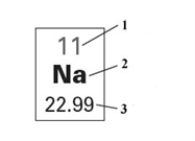 Which of the following correctly identifies the quantities labeled 1,2,and 3?
Which of the following correctly identifies the quantities labeled 1,2,and 3?
A)1 - atomic mass, 2 - element name, 3 - mass number
B)1 - atomic number, 2 - element symbol, 3 -atomic mass
C)1 - mass number , 2 - element symbol, 3 - atomic mass
D)1 - mass number, 2 - element name, 3 - atomic number
E)1 - atomic number, 2 - element symbol, 3 - mass number
 Which of the following correctly identifies the quantities labeled 1,2,and 3?
Which of the following correctly identifies the quantities labeled 1,2,and 3?A)1 - atomic mass, 2 - element name, 3 - mass number
B)1 - atomic number, 2 - element symbol, 3 -atomic mass
C)1 - mass number , 2 - element symbol, 3 - atomic mass
D)1 - mass number, 2 - element name, 3 - atomic number
E)1 - atomic number, 2 - element symbol, 3 - mass number

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is correct for the second period element in Group 5A/15?
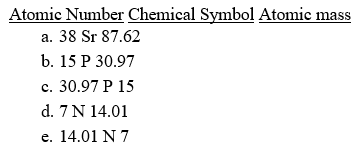

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the correct classification for both sodium and chlorine?
A)Na is a main group element and a metal and Cl is a main group element and a nonmetal.
B)Na is a main group element and a nonmetal and Cl is a main group element and a nonmetal.
C)Na is a main group element and a nonmetal and Cl is a main group element and a metal.
D)Na is a main group element and a metal and Cl is a main group element and a metal.
E)Na is a main group element and a metal and Cl is a transition element and a nonmetal.
A)Na is a main group element and a metal and Cl is a main group element and a nonmetal.
B)Na is a main group element and a nonmetal and Cl is a main group element and a nonmetal.
C)Na is a main group element and a nonmetal and Cl is a main group element and a metal.
D)Na is a main group element and a metal and Cl is a main group element and a metal.
E)Na is a main group element and a metal and Cl is a transition element and a nonmetal.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following figure which represents an element block on the periodic table. 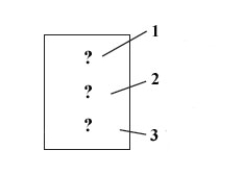 If this is the block for the nonmetal found in period 3 group 5A,the question marks 1,2,and 3 should be filled in,respectively,with which of the following?
If this is the block for the nonmetal found in period 3 group 5A,the question marks 1,2,and 3 should be filled in,respectively,with which of the following?
A)33, As, 74.92
B)23, V, 50.94
C)15, P, 30.97
D)39, Y, 88.91
 If this is the block for the nonmetal found in period 3 group 5A,the question marks 1,2,and 3 should be filled in,respectively,with which of the following?
If this is the block for the nonmetal found in period 3 group 5A,the question marks 1,2,and 3 should be filled in,respectively,with which of the following?A)33, As, 74.92
B)23, V, 50.94
C)15, P, 30.97
D)39, Y, 88.91

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following symbol/name pairs is/are correctly matched?
i.Fl,Fluorine
ii.N,Neon
iii.S,Sodium
iv.Ch,Chlorine
v.H,Hydrogen
A)i, ii, and v
B)i, iii, and v
C)i and v
D)iv and v
E)v only
i.Fl,Fluorine
ii.N,Neon
iii.S,Sodium
iv.Ch,Chlorine
v.H,Hydrogen
A)i, ii, and v
B)i, iii, and v
C)i and v
D)iv and v
E)v only

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the correct classification for both calcium and iron?
A)Ca is a transition element and a metal and Fe is a transition element and a metal.
B)Ca is a transition element and a metal and Fe is a transition element and a nonmetal.
C)Ca is a main group element and a metal and Fe is a transition element and a nonmetal.
D)Ca is a main group element and a metal and Fe is a transition element and a metal.
E)Ca is a main group element and a nonmetal and Fe is a main group element and a metal.
A)Ca is a transition element and a metal and Fe is a transition element and a metal.
B)Ca is a transition element and a metal and Fe is a transition element and a nonmetal.
C)Ca is a main group element and a metal and Fe is a transition element and a nonmetal.
D)Ca is a main group element and a metal and Fe is a transition element and a metal.
E)Ca is a main group element and a nonmetal and Fe is a main group element and a metal.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


