Exam 5: Atomic Theory: The Nuclear Model of the Atom
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations48 Questions
Exam 4: Introduction to Gases56 Questions
Exam 5: Atomic Theory: The Nuclear Model of the Atom48 Questions
Exam 6: Chemical Nomenclature45 Questions
Exam 7: Chemical Formula Relationships45 Questions
Exam 8: Chemical Reactions44 Questions
Exam 9: Chemical Change52 Questions
Exam 10: Quantity Relationships in Chemical Reactions42 Questions
Exam 11: Atomic Theory: The Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications47 Questions
Exam 15: Gases, Liquids, and Solids45 Questions
Exam 16: Solutions47 Questions
Exam 17: Acidbase Proton Transferreactions45 Questions
Exam 18: Chemical Equilibrium45 Questions
Exam 19: Oxidationreduction Electron Transferreactions45 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Examine the block on the periodic table indicated by the arrow. 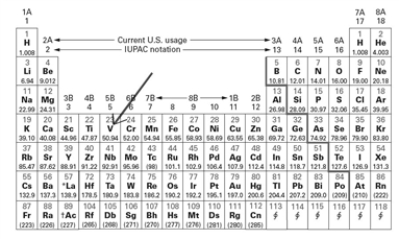 Which of the following correctly describes this element (V - vanadium)?
Which of the following correctly describes this element (V - vanadium)?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Which of the following statements is false?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
D
Which of the following gives the correct number of elements in each of the first six periods in the periodic table?
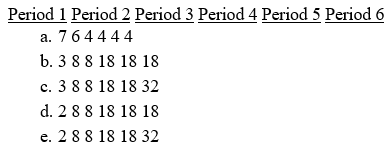
Free
(Short Answer)
4.8/5  (40)
(40)
Correct Answer:
E
Groups in the periodic table are also known as...
A)periods
B)IUPACs
C)Mendeleevian elements
D)chemical families
E)assemblages
(Short Answer)
4.9/5  (35)
(35)
Consider the following model of a generic atom. 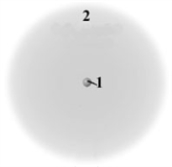 Which of the following is correct regarding the "regions" labeled 1 and 2?
Which of the following is correct regarding the "regions" labeled 1 and 2?
(Multiple Choice)
4.9/5  (29)
(29)
The nuclear symbol of an isotope of beryllium is 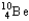 .Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.
.Indicate the number of protons,number of neutrons,atomic number,and mass number of the isotope.

(Short Answer)
4.8/5  (33)
(33)
The chemical symbol of germanium is Ge.An isotope of this element has 32 protons and 46 neutrons in the nucleus.What are the nuclear symbol,the atomic number,and the mass number of this isotope of germanium?
Nuclear symbol Atomic number Mass number
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following symbol/name pairs is/are correctly matched?
i.Fl,Fluorine
ii.N,Neon
iii.S,Sodium
iv.Ch,Chlorine
v.H,Hydrogen
(Multiple Choice)
4.9/5  (35)
(35)
Isotopes of an element vary in mass because of differing numbers of:
(Multiple Choice)
4.9/5  (34)
(34)
One of the features of Dalton's atomic theory no longer considered to be correct is: All atoms of each element are identical in every respect.Which of the following is the best explanation of why this feature is no longer considered valid?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is not a description of atoms according to Dalton's atomic theory?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is the best definition of the atomic mass unit?
(Multiple Choice)
4.9/5  (50)
(50)
Calculate the atomic mass of the element having the following distribution of isotopes:
5.8% 53.9396 u
91.8% 55.9349 u
2.1% 56.9354 u
0.3% 57.9333 u
(Multiple Choice)
4.8/5  (36)
(36)
Consider the following generic picture of an atom. 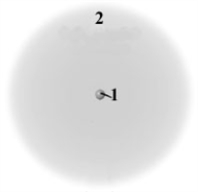 Which of the following is correct about the regions labeled 1 and 2?
Which of the following is correct about the regions labeled 1 and 2?
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following masses is closest to the mass of one atomic mass unit?
(Multiple Choice)
4.7/5  (36)
(36)
Dalton's atomic theory explained the observation that the percentage by mass of the elements in a compound is always the same,thus Dalton's atomic theory supports what Law?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is an incorrect statement about the Rutherford scattering experiment?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is not one of the main features of Dalton's atomic theory?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is the best explanation of why the mass of an atom is usually expressed in atomic mass units rather than grams?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 48
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)