Deck 32: Nuclear Physics and Nuclear Radiation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 32: Nuclear Physics and Nuclear Radiation
1
All nuclei of a given element have the same number of neutrons.
False
2
A fusion reaction generates energy by combining lighter nuclei into a heavier nucleus, plus additional particles.
True
3
Quarks can only have a charge of +(2/3)e or -(1/3)e, while antiquarks can only have charges of -(2/3)e or +(1/3)e.
True
4
The density of a nucleus (to a reasonable approximation) is independent of the mass number.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
The Sun is powered by the proton-proton cycle.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
The competition between the electrostatic force between the protons in a nucleus and the strong nuclear force determines whether a nucleus is stable.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
Particles made up of two quarks must consist of a quark-antiquark pair.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
Gravity waves are produced when a mass accelerates.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
The radius of a nucleus is proportional to what power of the mass number?
A) 1/2
B) 1/3
C) 1/4
D) 2/5
E) 3/8
A) 1/2
B) 1/3
C) 1/4
D) 2/5
E) 3/8

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
One type of event that may generate gravity waves of sufficient strength to be detected with current equipment is the death spiral of a neutron star as it plunges into a black hole.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
A moderator is inserted into a fission reaction to absorb neutrons and prevent additional fissions.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
The nuclei of different isotopes of a given element have the same number of
A) protons.
B) neutrons.
C) nucleons.
D) electrons.
E) positrons.
A) protons.
B) neutrons.
C) nucleons.
D) electrons.
E) positrons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
Photons associated with the magnetic field used in magnetic resonance imaging have considerably less energy than the photons used in CT scans.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
The proton is slightly more massive than the neutron.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
The force between two quarks increases with separation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
What combination of quarks produces a proton and what are the electric charges on these quarks, expressed in terms of e?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
The atomic number is equal to the number of what particles in the nucleus?
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
The mass number is equal to the number of what particles in the nucleus?
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.
A) protons
B) neutrons
C) nucleons
D) electrons
E) positrons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
The strong nuclear force acts only on neutral particles.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
What combination of quarks produces a neutron and what are the electric charges on these quarks, expressed in terms of e?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
A β- decay occurs in an unstable nucleus when
A) a proton is converted to an electron by the strong force.
B) a proton is converted to a neutron by the strong force.
C) a neutron is converted to a proton by the weak force.
D) a neutron is converted to an alpha particle by the weak force.
E) a neutron is converted to a beta particle by the weak force.
A) a proton is converted to an electron by the strong force.
B) a proton is converted to a neutron by the strong force.
C) a neutron is converted to a proton by the weak force.
D) a neutron is converted to an alpha particle by the weak force.
E) a neutron is converted to a beta particle by the weak force.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
The symbol for deuterium is 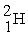 . How many protons are there in a deuterium nucleus?
. How many protons are there in a deuterium nucleus?
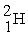 . How many protons are there in a deuterium nucleus?
. How many protons are there in a deuterium nucleus?
Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
When an unstable nucleus decays by emitting an alpha particle, the atomic number of the nucleus
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
The weakest of the four fundamental forces is
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.
A) the weak nuclear force.
B) the electromagnetic force.
C) the gravitational force.
D) the strong nuclear force.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
In beta minus decay, the number of protons in the nucleus is
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
In beta decay, it is found that the energy of the emitted electrons varies and is always less than what is calculated on the basis of the parent and daughter nuclei. This is evidence for the existence of another particle called the
A) gluon.
B) meson.
C) neutrino.
D) quark.
E) positrons.
A) gluon.
B) meson.
C) neutrino.
D) quark.
E) positrons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
In beta minus decay, the number of neutrons in the nucleus is
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.
A) decreased by 1.
B) decreased by 2.
C) increased by 1.
D) increased by 2.
E) remains unchanged.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
Composite particles that are composed of three quarks are called
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
When an unstable nucleus decays by emitting an alpha particle, the atomic mass number of the nucleus
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.
A) increases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 4.
E) remains unchanged.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
Elementary particles that experience the weak nuclear force but not the strong force are called
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
The nuclei of 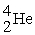 are also known as
are also known as
A) α particles.
B) β particles.
C) γ rays.
D) X-rays.
E) K-rays.
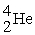 are also known as
are also known asA) α particles.
B) β particles.
C) γ rays.
D) X-rays.
E) K-rays.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
What type of scan is used to find the areas of the body where glucose metabolism is most intense?
A) PET scans
B) CT scans
C) MRI
D) radioactive iodine tracers
A) PET scans
B) CT scans
C) MRI
D) radioactive iodine tracers

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
One of the particle groups listed below includes two other groups on the list. Which group is it?
A) leptons
B) hadrons
C) mesons
D) bosons.
E) baryons
A) leptons
B) hadrons
C) mesons
D) bosons.
E) baryons

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
What are the mass number and the atomic number for each of the following:
(a) proton?
(b) neutron?
(c) alpha particle?
(a) proton?
(b) neutron?
(c) alpha particle?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
Polonium-216 decays to lead-212 by emitting what kind of nuclear radiation?
A) Alpha
B) Beta minus
C) Beta plus
D) Gamma
E) X-rays.
A) Alpha
B) Beta minus
C) Beta plus
D) Gamma
E) X-rays.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
Scientists hope that some day they will be able to produce a theory which will combine the strong force, the weak nuclear force, and electromagnetism. This theory has been called
A) The Grand Unified Theory.
B) The Theory of Everything.
C) The Unified Field Theory.
D) The Electroweak Theory.
E) The Electrostrong Theory.
A) The Grand Unified Theory.
B) The Theory of Everything.
C) The Unified Field Theory.
D) The Electroweak Theory.
E) The Electrostrong Theory.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
A fusion reaction works because the binding energy of the resulting nucleus
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
A fission reaction in which more neutrons are given off than are needed to initiate the reaction is called
A) a critical reaction.
B) a chain reaction.
C) a fusion reaction.
D) a neutron catastrophe.
E) a chemical reaction.
A) a critical reaction.
B) a chain reaction.
C) a fusion reaction.
D) a neutron catastrophe.
E) a chemical reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
Francium-223 decays to radium-223 by emitting what kind of nuclear radiation?
A) Alpha
B) Beta minus
C) Beta plus
D) Gamma
E) X-rays.
A) Alpha
B) Beta minus
C) Beta plus
D) Gamma
E) X-rays.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
Composite particles that are composed of two quarks are called
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.
A) leptons.
B) hadrons.
C) mesons.
D) bosons.
E) baryons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
Consider a particle composed of quarks.
(a) The negative pion is composed of what combination of up and down quarks?
(b) Is the negative pion a baryon or meson?
(c) Is the negative pion a lepton or a hadron?
(a) The negative pion is composed of what combination of up and down quarks?
(b) Is the negative pion a baryon or meson?
(c) Is the negative pion a lepton or a hadron?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
Write the nuclear notation for the following nuclei:
Hydrogen-2, Sulfur-33, and Lead-207.
Hydrogen-2, Sulfur-33, and Lead-207.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
A radioactive substance containing 4.00 × 1016 unstable atoms has a half-life of 2.0 days. What is its activity (in curies) after 1 day?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
What is the approximate nuclear radius of an isotope of sodium with 11 protons and 12 neutrons?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
The activity of a sample reduces from 7.55 × 109 Bq to 5.11 × 109 Bq in 5.50 months.
(a) What is the half life?
(b) How many months must one wait for the activity to decrease to 10%?
(a) What is the half life?
(b) How many months must one wait for the activity to decrease to 10%?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
Complete the following nuclear decay equation:
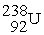 →
→ 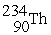 + ________
+ ________
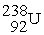 →
→ 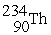 + ________
+ ________
Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
Suppose that in a certain collection of nuclei there were initially 1024 nuclei and 20 minutes later there was only one nuclei left, the others having decayed. On the basis of this information, how many nuclei would you estimate decayed in the first 6 minutes?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
Polonium-215 alpha decays with a half life of 1.8 ms.
(a) How much is left after 1. second?
(b) What is the daughter nucleus?
(a) How much is left after 1. second?
(b) What is the daughter nucleus?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
The symbol for a certain isotope of polonium is 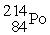 . How many neutrons are there in the nucleus of this isotope?
. How many neutrons are there in the nucleus of this isotope?
A) 84
B) 130
C) 214
D) 298
E) 314
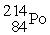 . How many neutrons are there in the nucleus of this isotope?
. How many neutrons are there in the nucleus of this isotope?A) 84
B) 130
C) 214
D) 298
E) 314

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
Complete the following nuclear decay equation:
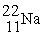 + ________ →
+ ________ → 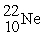
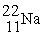 + ________ →
+ ________ → 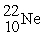

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
An old bone sample contains 321. grams of carbon and its activity is 947. decays/min.
(5730. yr half life of Carbon-14)
(a) How many thousand years old is the sample?
(b) What will be the activity of the sample 2000. yrs from now?
(5730. yr half life of Carbon-14)
(a) How many thousand years old is the sample?
(b) What will be the activity of the sample 2000. yrs from now?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
One mole of Strontium-90 is decaying with a half life of 28. years. After 61. years, how much Strontium is left and what is its activity?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
The symbol for a certain isotope of radium is 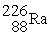 . How many nucleons are there in the nucleus of this isotope?
. How many nucleons are there in the nucleus of this isotope?
A) 88
B) 138
C) 214
D) 226
E) 314
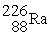 . How many nucleons are there in the nucleus of this isotope?
. How many nucleons are there in the nucleus of this isotope?A) 88
B) 138
C) 214
D) 226
E) 314

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
If the "half life" for Radium-226 is 1600. years, how long is the "quarter life"?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
If a nuclide has a decay constant of 0.765 × 10-6 s-1 and contains 3.0 × 1017 nuclei
(a) what is the half life?
(b) what is its initial activity?
(c) what is the activity after 12. days?
(a) what is the half life?
(b) what is its initial activity?
(c) what is the activity after 12. days?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
The symbol for a certain isotope of strontium is 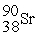 . What is the mass number of this isotope?
. What is the mass number of this isotope?
A) 38
B) 52
C) 88
D) 90
E) 128
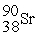 . What is the mass number of this isotope?
. What is the mass number of this isotope?A) 38
B) 52
C) 88
D) 90
E) 128

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
How much energy is required to remove one proton from 9Be?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
What are the mass number and the atomic number for each of the following:
(a) beta+?
(b) beta-?
(a) beta+?
(b) beta-?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the fission reaction:
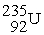 +
+ 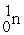 → 2 fission products + 2 n's
→ 2 fission products + 2 n's
(a) Estimate the energy released in the reaction.
(b) If 3 neutrons had been released instead of 2, what energy would have been released?
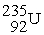 +
+ 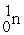 → 2 fission products + 2 n's
→ 2 fission products + 2 n's(a) Estimate the energy released in the reaction.
(b) If 3 neutrons had been released instead of 2, what energy would have been released?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
A sodium-22 nucleus ( 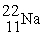 ) decays into a neon-22 nucleus (
) decays into a neon-22 nucleus ( 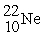 ) and one other particle. What is the other particle?
) and one other particle. What is the other particle?
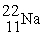 ) decays into a neon-22 nucleus (
) decays into a neon-22 nucleus ( 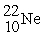 ) and one other particle. What is the other particle?
) and one other particle. What is the other particle?
Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
Rutherfordium-261 has a half-life of 1.08 min. How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
A) 1.02 min
B) 1.62 min
C) 0.63 min
D) 2.70 min
E) 3.24 min
A) 1.02 min
B) 1.62 min
C) 0.63 min
D) 2.70 min
E) 3.24 min

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
What is the density of the nucleus of 90Sr?
A) greater than 3 × 1020 kg/m3
B) 90 kg/m3
C) 2.1 × 1019 kg/m3
D) 2.0 × 1015 kg/m3
E) 2.3 × 1017 kg/m3
A) greater than 3 × 1020 kg/m3
B) 90 kg/m3
C) 2.1 × 1019 kg/m3
D) 2.0 × 1015 kg/m3
E) 2.3 × 1017 kg/m3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
The neutral helium atom, 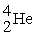 , has a mass of 4.002603 u, a neutron has a mass of 1.008665 u, a proton has a mass of 1.007277 u, and a neutral hydrogen atom has a mass of 1.007825 u. What is the binding energy of the
, has a mass of 4.002603 u, a neutron has a mass of 1.008665 u, a proton has a mass of 1.007277 u, and a neutral hydrogen atom has a mass of 1.007825 u. What is the binding energy of the 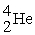 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.
A) 20.5 MeV
B) 22.4 MeV
C) 27.3 MeV
D) 28.3 MeV
E) 23.4 MeV
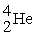 , has a mass of 4.002603 u, a neutron has a mass of 1.008665 u, a proton has a mass of 1.007277 u, and a neutral hydrogen atom has a mass of 1.007825 u. What is the binding energy of the
, has a mass of 4.002603 u, a neutron has a mass of 1.008665 u, a proton has a mass of 1.007277 u, and a neutral hydrogen atom has a mass of 1.007825 u. What is the binding energy of the 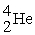 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.A) 20.5 MeV
B) 22.4 MeV
C) 27.3 MeV
D) 28.3 MeV
E) 23.4 MeV

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
Fermium-253 has a half-life of 3.00 days. A sample of fermium has 3.88 × 106 nuclei. What is the initial activity of this sample?
A) 10.4 Bq
B) 10.4 Ci
C) 12.9 Bq
D) 12.9 Ci
A) 10.4 Bq
B) 10.4 Ci
C) 12.9 Bq
D) 12.9 Ci

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
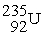 is radioactive and decays in a series to
is radioactive and decays in a series to 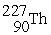 . In this series, the particles ejected from the nucleus are
. In this series, the particles ejected from the nucleus areA) one alpha particle and three beta particles.
B) three alpha particles and one beta particles.
C) one alpha particle and four beta particles.
D) four alpha particle and one beta particle.
E) two alpha particles and two beta particles.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
A certain substance has a half-life of 5.0 hours. How many nuclei of the substance are required to give an initial activity of 6.0 μCi? 1 Ci = 3.7 × 1010 Bq.
A) 5.8 × 109 nuclei
B) 8.5 × 108 nuclei
C) 6.3 × 108 nuclei
D) 3.2 × 109 nuclei
E) 2.4 × 109 nuclei
A) 5.8 × 109 nuclei
B) 8.5 × 108 nuclei
C) 6.3 × 108 nuclei
D) 3.2 × 109 nuclei
E) 2.4 × 109 nuclei

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
The neutral deuterium atom, 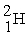 , has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 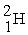 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.
A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV
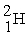 , has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 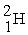 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
What is the nuclear radius of 90Sr?
A) 4.0 × 10-15 m
B) 1.2 × 10-15 m
C) 1.1 × 10-13 m
D) 5.4 × 10-15 m
E) 3.3 × 10-13 m
A) 4.0 × 10-15 m
B) 1.2 × 10-15 m
C) 1.1 × 10-13 m
D) 5.4 × 10-15 m
E) 3.3 × 10-13 m

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
The number of radioactive nuclei in a particular sample decreases to one-eighth of its original number in 9 days. What is the half-life of these nuclei?
A) 9/8 days
B) 2 days
C) 3 days
D) 8 days
E) 10 days
A) 9/8 days
B) 2 days
C) 3 days
D) 8 days
E) 10 days

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
An electron and a positron annihilate each other, emitting two identical photons in the process. What is the wavelength of these photons? The mass of an electron or positron is 9.11 × 10-31 kg and h = 6.63 × 10-34 Js.
A) 1.21 × 10-12 m
B) 1.73 × 10-12 m
C) 2.43 × 10-12 m
D) 3.07 × 10-12 m
E) 3.46 × 10-12 m
A) 1.21 × 10-12 m
B) 1.73 × 10-12 m
C) 2.43 × 10-12 m
D) 3.07 × 10-12 m
E) 3.46 × 10-12 m

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are:
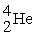 , 4.002603 u;
, 4.002603 u; 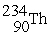 , 234.043583 u;
, 234.043583 u; 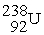 , 238.050786 u.
, 238.050786 u.
A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV
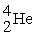 , 4.002603 u;
, 4.002603 u; 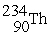 , 234.043583 u;
, 234.043583 u; 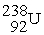 , 238.050786 u.
, 238.050786 u.A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
The decay constant of radon is 0.181 day s-1. A sample of radon contains 6.00 × 108 radon atoms. How many atoms are left after 10.0 days?
A) 9.82 × 107
B) 7.67 × 107
C) 8.34 × 108
D) 7.29 × 108
E) 8.56 × 108
A) 9.82 × 107
B) 7.67 × 107
C) 8.34 × 108
D) 7.29 × 108
E) 8.56 × 108

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
Americium-243 has a decay constant of 9.39 × 10-5 years-1. How long will it take for a sample of americium to lose one-third of its nuclei?
A) 2960 s
B) 10.6 × 103 years
C) 4.32 × 103 years
D) 6.44 × 103 years
E) 7.84 × 103 years
A) 2960 s
B) 10.6 × 103 years
C) 4.32 × 103 years
D) 6.44 × 103 years
E) 7.84 × 103 years

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are:
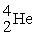 , 4.002603 u;
, 4.002603 u; 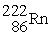 , 222.017570 u;
, 222.017570 u; 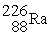 , 226.025402 u.
, 226.025402 u.
A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV
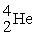 , 4.002603 u;
, 4.002603 u; 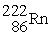 , 222.017570 u;
, 222.017570 u; 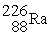 , 226.025402 u.
, 226.025402 u.A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 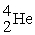 , 4.002603 u;
, 4.002603 u; 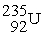 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 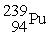 ?
?
1 u = 931.494 MeV/c2.
A) 239.052157 u
B) 239.027750 u
C) 239.001889 u
D) 238.9991889 u
E) 238.988842 u
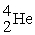 , 4.002603 u;
, 4.002603 u; 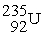 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 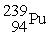 ?
?1 u = 931.494 MeV/c2.
A) 239.052157 u
B) 239.027750 u
C) 239.001889 u
D) 238.9991889 u
E) 238.988842 u

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
Carbon-14 has a half-life of 5730 years. A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory, where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with the activity of living organic matter, 0.230 Bq/g, the scientist determines how old the wood sample is, or more precisely, when the tree that the sample came from died. How old is the sample of wood?
A) 3870 years
B) 4250 years
C) 4590 years
D) 2630 years
E) 2940 years
A) 3870 years
B) 4250 years
C) 4590 years
D) 2630 years
E) 2940 years

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
Fermium-253 has a half-life of 3.00 days. A sample of fermium has 7.37 × 107 nuclei. How long will it take for there to be only 3.36 × 106 fermium nuclei in this sample?
A) 2.75 days
B) 9.80 days
C) 13.4 days
D) 15.7 days
E) 58.6 days
A) 2.75 days
B) 9.80 days
C) 13.4 days
D) 15.7 days
E) 58.6 days

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
An oxygen-15 nucleus ( 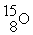 ) decays by emitting a β+ ray and one other particle. What is the other particle?
) decays by emitting a β+ ray and one other particle. What is the other particle?
A)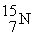
B)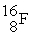
C)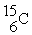
D)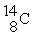
E)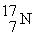
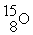 ) decays by emitting a β+ ray and one other particle. What is the other particle?
) decays by emitting a β+ ray and one other particle. What is the other particle?A)
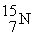
B)
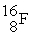
C)
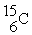
D)
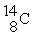
E)
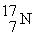

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 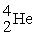 , 4.002603 u;
, 4.002603 u; 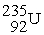 , 235.043924 u; what is the kinetic energy of the
, 235.043924 u; what is the kinetic energy of the 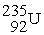 nucleus?
nucleus?
1 u =931.494 MeV/c2.
A) 0.0829 MeV
B) 0.0837 MeV
C) 0.0852 MeV
D) 0.0863 MeV
E) 0.0877 MeV
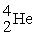 , 4.002603 u;
, 4.002603 u; 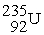 , 235.043924 u; what is the kinetic energy of the
, 235.043924 u; what is the kinetic energy of the 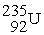 nucleus?
nucleus?1 u =931.494 MeV/c2.
A) 0.0829 MeV
B) 0.0837 MeV
C) 0.0852 MeV
D) 0.0863 MeV
E) 0.0877 MeV

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
Rutherfordium-261 has a half-life of 1.08 min. What is the decay constant of rutherfordium?
A) 0.0107 s-1
B) 0.0154 s-1
C) 1.87 s-1
D) 2.91 s-1
E) 0.344 s-1
A) 0.0107 s-1
B) 0.0154 s-1
C) 1.87 s-1
D) 2.91 s-1
E) 0.344 s-1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck


