Exam 32: Nuclear Physics and Nuclear Radiation
Exam 1: Introduction to Physics100 Questions
Exam 2: One-Dimensional Kinematics112 Questions
Exam 3: Vectors in Physics82 Questions
Exam 4: Two-Dimensional Kinematics95 Questions
Exam 5: Newtons Laws of Motion101 Questions
Exam 6: Applications of Newtons Laws105 Questions
Exam 7: Work and Kinetic Energy92 Questions
Exam 8: Potential Energy and Conservation of Energy99 Questions
Exam 9: Linear Momentum and Collisions102 Questions
Exam 10: Rotational Kinematics and Energy102 Questions
Exam 11: Rotational Dynamics and Static Equilibrium97 Questions
Exam 12: Gravity94 Questions
Exam 13: Oscillations About Equilibrium102 Questions
Exam 14: Waves and Sound104 Questions
Exam 15: Fluids107 Questions
Exam 16: Temperature and Heat103 Questions
Exam 17: Phases and Phase Changes100 Questions
Exam 18: The Laws of Thermodynamics97 Questions
Exam 19: Electric Charges, Forces, and Fields88 Questions
Exam 20: Electric Potential and Electric Potential Energy99 Questions
Exam 21: Electric Current and Direct-Current Circuits99 Questions
Exam 22: Magnetism101 Questions
Exam 23: Magnetic Flux and Faradays Law of Induction99 Questions
Exam 24: Alternating-Current Circuits93 Questions
Exam 25: Electromagnetic Waves90 Questions
Exam 26: Geometrical Optics92 Questions
Exam 27: Optical Instruments102 Questions
Exam 28: Physical Optics: Interference and Diffraction93 Questions
Exam 29: Relativity100 Questions
Exam 30: Quantum Physics100 Questions
Exam 31: Atomic Physics75 Questions
Exam 32: Nuclear Physics and Nuclear Radiation89 Questions
Select questions type
In beta minus decay, the number of neutrons in the nucleus is
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
A
An electron and a positron annihilate each other, emitting two identical photons in the process. What is the wavelength of these photons? The mass of an electron or positron is 9.11 × 10-31 kg and h = 6.63 × 10-34 Js.
Free
(Multiple Choice)
5.0/5  (40)
(40)
Correct Answer:
C
A moderator is inserted into a fission reaction to absorb neutrons and prevent additional fissions.
Free
(True/False)
4.8/5  (39)
(39)
Correct Answer:
False
An old bone sample contains 321. grams of carbon and its activity is 947. decays/min.
(5730. yr half life of Carbon-14)
(a) How many thousand years old is the sample?
(b) What will be the activity of the sample 2000. yrs from now?
(Short Answer)
4.9/5  (33)
(33)
The competition between the electrostatic force between the protons in a nucleus and the strong nuclear force determines whether a nucleus is stable.
(True/False)
4.7/5  (32)
(32)
Composite particles that are composed of three quarks are called
(Multiple Choice)
4.7/5  (25)
(25)
Fermium-253 has a half-life of 3.00 days. A sample of fermium has 3.88 × 106 nuclei. What is the initial activity of this sample?
(Multiple Choice)
4.9/5  (35)
(35)
Elementary particles that experience the weak nuclear force but not the strong force are called
(Multiple Choice)
4.7/5  (27)
(27)
Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are:
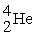 , 4.002603 u;
, 4.002603 u; 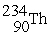 , 234.043583 u;
, 234.043583 u; 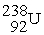 , 238.050786 u.
, 238.050786 u.
(Multiple Choice)
4.8/5  (38)
(38)
What combination of quarks produces a proton and what are the electric charges on these quarks, expressed in terms of e?
(Essay)
4.9/5  (35)
(35)
Composite particles that are composed of two quarks are called
(Multiple Choice)
4.8/5  (27)
(27)
When a neutron collides with a uranium-235 nucleus it can induce a variety of fission reactions. One such reaction is 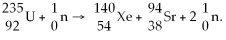 Given the following mass values:
Given the following mass values: 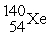 , 139.921620 u;
, 139.921620 u; 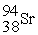 , 93.915367 u;
, 93.915367 u; 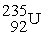 , 235.043924 u;
, 235.043924 u; 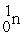 , 1.008665 u, what mass of uranium is needed to produce a 10-kiloton yield?
1 u = 1.660540 × 10-27 kg; 1 kiloton = 5.0 × 1012 J.
, 1.008665 u, what mass of uranium is needed to produce a 10-kiloton yield?
1 u = 1.660540 × 10-27 kg; 1 kiloton = 5.0 × 1012 J.
(Multiple Choice)
4.8/5  (34)
(34)
The decay constant of radon is 0.181 day s-1. A sample of radon contains 6.00 × 108 radon atoms. How many atoms are left after 10.0 days?
(Multiple Choice)
4.9/5  (44)
(44)
Consider a particle composed of quarks.
(a) The negative pion is composed of what combination of up and down quarks?
(b) Is the negative pion a baryon or meson?
(c) Is the negative pion a lepton or a hadron?
(Short Answer)
4.9/5  (36)
(36)
If a reactor produces an average power of 1000 MW for a year, how much 235U is used up assuming 200 MeV are released per fission?
(Multiple Choice)
4.9/5  (35)
(35)
A fission reaction in which more neutrons are given off than are needed to initiate the reaction is called
(Multiple Choice)
4.8/5  (34)
(34)
The symbol for a certain isotope of strontium is 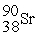 . What is the mass number of this isotope?
. What is the mass number of this isotope?
(Multiple Choice)
4.8/5  (43)
(43)
Polonium-216 decays to lead-212 by emitting what kind of nuclear radiation?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)