Deck 2: Basic Chemistry of Cells
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 2: Basic Chemistry of Cells
1
Which factor determines whether an atom will be chemically reactive?
A)the number of electrons in the outer shell
B)the ratio of protons to electrons
C)the number of electrons in the inner shell
D)the ratio of protons to neutrons
E)the number of electron shells an atom has
A)the number of electrons in the outer shell
B)the ratio of protons to electrons
C)the number of electrons in the inner shell
D)the ratio of protons to neutrons
E)the number of electron shells an atom has
A
Explanation: The number of electrons in an atom's outer shell,called the valence shell,determines its chemical reactivity.
Explanation: The number of electrons in an atom's outer shell,called the valence shell,determines its chemical reactivity.
2
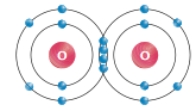
This diagram shows that two oxygen atoms interact
A)to form one covalent bond.
B)to form two covalent bonds.
C)to form four covalent bonds.
D)to form an ionic bonD.
E)to form hydrogen bonds.
B
Explanation: A double covalent bond occurs when two atoms share two pairs of electrons.
Explanation: A double covalent bond occurs when two atoms share two pairs of electrons.
3
A chemist found one atom with 9 protons,8 neutrons and 7 electrons.Another atom has 8 protons,9 neutrons and 10 electrons.Which of the following statements is correct?
A)Both atoms have the same mass number.
B)Both atoms have -2 electrical charge.
C)Both atoms are isotopes of the same element.
D)Both atoms are chemically non-reactive and would not interact with other atoms.
E)Both atoms have fulfilled the octet rulE.
A)Both atoms have the same mass number.
B)Both atoms have -2 electrical charge.
C)Both atoms are isotopes of the same element.
D)Both atoms are chemically non-reactive and would not interact with other atoms.
E)Both atoms have fulfilled the octet rulE.
A
Explanation: All atoms of an element have the same number of protons.The mass number is the sum of the number of protons and the number of neutrons.The charge atom is determined by the number of electrons and protons.Atoms with 8 electrons filling the outer shell are following the octet rule.
Explanation: All atoms of an element have the same number of protons.The mass number is the sum of the number of protons and the number of neutrons.The charge atom is determined by the number of electrons and protons.Atoms with 8 electrons filling the outer shell are following the octet rule.
4
Which of these describes the structure of an atom?
A)Positive protons and negative electrons in the nucleus are surrounded by neutral neutrons.
B)Neutral protons and negative neutrons in the nucleus are surrounded by positive electrons.
C)Positive protons and neutral neutrons in the nucleus are surrounded by negative electrons.
D)Negative protons and positive neutrons in the nucleus are surrounded by neutral electrons.
E)Positive protons and negative neutrons in the nucleus are surrounded by neutral electrons.
A)Positive protons and negative electrons in the nucleus are surrounded by neutral neutrons.
B)Neutral protons and negative neutrons in the nucleus are surrounded by positive electrons.
C)Positive protons and neutral neutrons in the nucleus are surrounded by negative electrons.
D)Negative protons and positive neutrons in the nucleus are surrounded by neutral electrons.
E)Positive protons and negative neutrons in the nucleus are surrounded by neutral electrons.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
A neutral atom of phosphorus was found to have an atomic number of 15 and a mass number of 31.What is the total number of electrons in this atom?
A)16
B)15
C)31
D)8
E)46
A)16
B)15
C)31
D)8
E)46

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
When the number of protons does not equal the number of electrons,the atom is called
A)an isotope
B)an ion
C)a compound
D)an octet
E)a valance
A)an isotope
B)an ion
C)a compound
D)an octet
E)a valance

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is positively charged?
A)proton
B)electron
C)atomic mass
D)neutron
E)isotope
A)proton
B)electron
C)atomic mass
D)neutron
E)isotope

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following elements is important in smaller quantities than is true for the six major elements in living things?
A)carbon
B)iron
C)oxygen
D)nitrogen
E)hydrogen
A)carbon
B)iron
C)oxygen
D)nitrogen
E)hydrogen

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
The number of protons found in an atom is also known as:
A)electron number
B)isotope number
C)neutron number
D)atomic number
E)atomic mass
A)electron number
B)isotope number
C)neutron number
D)atomic number
E)atomic mass

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
A sulfur atom has 6 electrons in the outer electron shell.What will it most likely do?
A)gain two electrons from another atom
B)lose 6 neutrons to another atom
C)nothing,as this is a very stable atom
D)lose two electrons to another atom
E)stay as a single atom in nature
A)gain two electrons from another atom
B)lose 6 neutrons to another atom
C)nothing,as this is a very stable atom
D)lose two electrons to another atom
E)stay as a single atom in nature

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
The identity of any atom can be determined by the
A)number of electrons.
B)atomic charge.
C)atomic mass.
D)number of protons.
E)number of neutrons.
A)number of electrons.
B)atomic charge.
C)atomic mass.
D)number of protons.
E)number of neutrons.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
In order to determine if a patient has a thyroid tumor,which diagnostic procedure could be performed?
A)Patient drinks low levels of radioactive Iodine-131,then has an X-ray.
B)
Patient drinks high levels of radioactive Iodine-131,then has a PET scan.
C)Injection of low levels of radioactive glucose,then patient has a PET scan.
D)Injection of low levels of radioactive Thallium-201,then patient has a PET sca.n
E)Injection of high levels of radioactive glucose,then patient has an X-ray.
A)Patient drinks low levels of radioactive Iodine-131,then has an X-ray.
B)
Patient drinks high levels of radioactive Iodine-131,then has a PET scan.
C)Injection of low levels of radioactive glucose,then patient has a PET scan.
D)Injection of low levels of radioactive Thallium-201,then patient has a PET sca.n
E)Injection of high levels of radioactive glucose,then patient has an X-ray.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following would be the largest number describing an atom?
A)Electron number
B)Neutron number
C)Atomic number
D)Proton number
E)Mass number
A)Electron number
B)Neutron number
C)Atomic number
D)Proton number
E)Mass number

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
How is the mass number of an element determined?
A)the number of neutrons plus the number of electrons
B)the number of electrons only
C)the number of protons plus the number of neutrons
D)the number of protons plus the number of electrons
E)the number of protons only
A)the number of neutrons plus the number of electrons
B)the number of electrons only
C)the number of protons plus the number of neutrons
D)the number of protons plus the number of electrons
E)the number of protons only

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Which six elements are the main components in living organisms?
A)carbon,hydrogen,nitrogen,oxygen,phosphorus,sulfur
B)copper,iron,magnesium,sodium,water,zinc
C)carbon dioxide,hydrogen,nitrogen,oxygen,phosphate,sulfate
D)calcium,hydrogen,iron,potassium,sulfur,water
E)aluminum,magnesium,nitrogen,silicon,sodium,sulfur
A)carbon,hydrogen,nitrogen,oxygen,phosphorus,sulfur
B)copper,iron,magnesium,sodium,water,zinc
C)carbon dioxide,hydrogen,nitrogen,oxygen,phosphate,sulfate
D)calcium,hydrogen,iron,potassium,sulfur,water
E)aluminum,magnesium,nitrogen,silicon,sodium,sulfur

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
Although high doses of radiation are harmful to cells,how can low levels of radioactive isotopes be used medicinally?
A)as tracers in imaging organs using Xrays and PET Scans
B)to reduce obesity and diabetes in teenagers
C)to create drugs that work faster than normal
D)to destroy aging and unwanted cells
E)to prevent ultraviolet damage from the sun
A)as tracers in imaging organs using Xrays and PET Scans
B)to reduce obesity and diabetes in teenagers
C)to create drugs that work faster than normal
D)to destroy aging and unwanted cells
E)to prevent ultraviolet damage from the sun

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following changes would cause an atom to become a different element?
A)increase the number of neutrons
B)increase the number of electrons
C)increase the number of protons
D)decrease the number of neutrons
E)decrease the number of electrons
A)increase the number of neutrons
B)increase the number of electrons
C)increase the number of protons
D)decrease the number of neutrons
E)decrease the number of electrons

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
What is different between two isotopes of the same element?
A)valance number
B)proton number
C)mass number
D)atomic number
E)atomic charge
A)valance number
B)proton number
C)mass number
D)atomic number
E)atomic charge

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
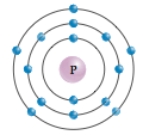
This diagram shows an atom of phosphorus.How many electrons are in the valence shell?
A)2
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
If a neutral oxygen atom gains two electrons from another atom,then the overall charge of this oxygen atom will become
A)+2
B)0
C)-2
D)unknown,as you need to know how many protons are present
E)2 more than its original mass number
A)+2
B)0
C)-2
D)unknown,as you need to know how many protons are present
E)2 more than its original mass number

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Which interaction bonds water molecules to other water molecules?
A)hydrophobic interaction
B)hydrogen bond
C)ionic bond
D)covalent bond
E)adhesive interaction
A)hydrophobic interaction
B)hydrogen bond
C)ionic bond
D)covalent bond
E)adhesive interaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
Which term describes the tendency of water molecules to cling to other water molecules?
A)adhesion
B)electronegativity
C)polar
D)ccohesion
E)nonpolar
A)adhesion
B)electronegativity
C)polar
D)ccohesion
E)nonpolar

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
When you mix sugar into your coffee,the sugar dissolves in the liquid.This shows that sugar molecules are
A)hydrophilic.
B)cohesive.
C)covalent.
D)neither hydrophobic nor hydrophilic.
E)hydrophobic.
A)hydrophilic.
B)cohesive.
C)covalent.
D)neither hydrophobic nor hydrophilic.
E)hydrophobic.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
Certain insects,such as a water strider,can walk across the surface of a pond.Which property of water allows this?
A)Frozen water is less dense than liquid water.
B)Water molecules are able to stick to other water molecules.
C)Water can dissolve polar and ionic compounds.
D)Water molecules are able to stick to other non-water molecules.
E)Water repels hydrophobic materials.
A)Frozen water is less dense than liquid water.
B)Water molecules are able to stick to other water molecules.
C)Water can dissolve polar and ionic compounds.
D)Water molecules are able to stick to other non-water molecules.
E)Water repels hydrophobic materials.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
Which chemical bond results from the equal sharing of electrons between two atoms?
A)polar
B)hydrogen
C)ionic
D)nonpolar
E)adhesive
A)polar
B)hydrogen
C)ionic
D)nonpolar
E)adhesive

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
Which kind of bond occurs between positively and negatively charged atoms?
A)covalent bond
B)adhesive interaction
C)hydrogen bond
D)ionic bond
E)hydrophobic interaction
A)covalent bond
B)adhesive interaction
C)hydrogen bond
D)ionic bond
E)hydrophobic interaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
What kind of interaction is shown between sodium and chlorine? 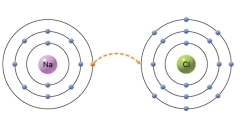
A)The sodium atom gives up one proton.
B)The sodium atom gives up one neutron.
C)The chlorine atom gives up one neutron.
D)The chlorine atom gives up one electron.
E)The sodium atom gives up one electron.

A)The sodium atom gives up one proton.
B)The sodium atom gives up one neutron.
C)The chlorine atom gives up one neutron.
D)The chlorine atom gives up one electron.
E)The sodium atom gives up one electron.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
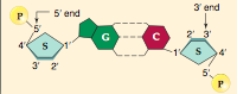
In a DNA molecule,the polar "G" nucleotide on one strand always bonds with the polar "C" nucleotide on the other strand.What kind of bonds are these?
A)hydrophobic interactions
B)hydrogen bonds
C)adhesive interactions
D)covalent bonds
E)ionic bonds

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
What term describes the tendency of water molecules to cling to the wall of a blood vessel?
A)hydrophobicity
B)buffering
C)cohesion
D)adhesion
E)electronegativity
A)hydrophobicity
B)buffering
C)cohesion
D)adhesion
E)electronegativity

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
An unknown solution is poured into a beaker containing water and is stirred vigorously.After a few minutes,the scientist observes that the two liquids are not mixing.Based on this observation,what conclusion can the scientist make?
A)The unknown solution is adhesive.
B)The unknown solution is covalent.
C)The unknown solution is ioniC.
D)The unknown solution is hydrophilic.
E)The unknown solution is hydrophobic.
A)The unknown solution is adhesive.
B)The unknown solution is covalent.
C)The unknown solution is ioniC.
D)The unknown solution is hydrophilic.
E)The unknown solution is hydrophobic.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is not a use of water by living organisms?
A)external transportation for chemicals
B)help exchange heat
C)aids in homeostasis
D)provides nutrients for metabolism
E)provides a medium for movement
A)external transportation for chemicals
B)help exchange heat
C)aids in homeostasis
D)provides nutrients for metabolism
E)provides a medium for movement

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is not true about most other solvents,rather than about water ice?
A)It is less dense than liquid water
B)It insulates and slows down the freezing of water below
C)It freezes from top to bottom
D)It floats in water
E)It is denser than liquid water
A)It is less dense than liquid water
B)It insulates and slows down the freezing of water below
C)It freezes from top to bottom
D)It floats in water
E)It is denser than liquid water

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
When there in an unequal sharing of electrons between two atoms,what will be the result?
A)a nonpolar molecule,such as methane.
B)an ionic compound,such as salt.
C)an electronegative molecule,such as calcium ion.
D)a hydrophobic molecule,such as fat.
E)a polar molecule,such as water.
A)a nonpolar molecule,such as methane.
B)an ionic compound,such as salt.
C)an electronegative molecule,such as calcium ion.
D)a hydrophobic molecule,such as fat.
E)a polar molecule,such as water.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
When salt crystals dissolve in water,which chemical interactions in the salt crystals are being dissociated by the water molecules?
A)nonpolar covalent bonds
B)hydrogen bonds
C)polar covalent bonds
D)ionic bonds
E)hydrophobic interactions
A)nonpolar covalent bonds
B)hydrogen bonds
C)polar covalent bonds
D)ionic bonds
E)hydrophobic interactions

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
When water is mixed with a salt in a beaker,the salt would be considered
A)the solvent.
B)the solute.
C)the solution.
D)the buffer.
E)the ion.
A)the solvent.
B)the solute.
C)the solution.
D)the buffer.
E)the ion.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
Hydrochloric acid is classified as an acid because
A)it absorbs excess hydroxide ions from the solution.
B)it dissociates to release hydroxide ions.
C)it dissociates to release hydrogen ions and absorbs excess hydroxide ions from the solution.
D)it absorbs excess hydrogen ions from the solution.
E)it dissociates to release hydrogen ions.
A)it absorbs excess hydroxide ions from the solution.
B)it dissociates to release hydroxide ions.
C)it dissociates to release hydrogen ions and absorbs excess hydroxide ions from the solution.
D)it absorbs excess hydrogen ions from the solution.
E)it dissociates to release hydrogen ions.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
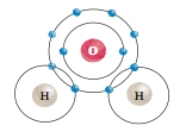
What type of chemical bond occurs specifically between one hydrogen atom and one oxygen atom in a water molecule?
A)a polar covalent bond
B)an ionic bond
C)hydrogen bond
D)a nonpolar covalent bond
E)a hydrophobic interaction

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Which ions found specifically in bones and teeth are important in muscle contractions and nerve conduction?
A)sodium
B)chloride
C)bicarbonate
D)potassium
E)calcium
A)sodium
B)chloride
C)bicarbonate
D)potassium
E)calcium

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
Which bond results from the sharing of electrons?
A)isotope
B)metallic
C)covalent
D)ionic
E)octet
A)isotope
B)metallic
C)covalent
D)ionic
E)octet

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
In a solution,if the hydroxide ion concentration exceeds the hydrogen ion concentration the solution is considered to be
A)a buffer.
B)an acid.
C)a neutral solution.
D)a base.
E)a solutE.
A)a buffer.
B)an acid.
C)a neutral solution.
D)a base.
E)a solutE.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
The bond which results from a transfer of electrons from one atom to another is called an ionic bond.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Lemon Juice has a pH of 2.3 and should be classified as a
A)strong acid.
B)buffering solution.
C)weak acid.
A)strong acid.
B)buffering solution.
C)weak acid.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
When the concentration of hydrogen ions is greater than the concentration of hydroxide ions the solution is considered an acid.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
Alkalosis,cramping,and irritability can occur when our blood pH rises above 7.45.Acidosis,seizures,coma and death can occur when our blood pH falls below pH 7.35.To prevent these conditions,our blood usually has chemicals which
A)can take up both excess hydrogen ions and excess hydroxide ions.
B)can produce more water molecules.
C)can take up excess hydroxide ions.
D)can take up excess hydrogen ions.
E)keep pH at neutral statE.
A)can take up both excess hydrogen ions and excess hydroxide ions.
B)can produce more water molecules.
C)can take up excess hydroxide ions.
D)can take up excess hydrogen ions.
E)keep pH at neutral statE.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
Two or more different elements bonded together is called an isotope.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is not an impact of acid on the environment?
A)hurts human health
B)damages buildings and other human made structures
C)pollution of surface water
D)destroys forests
E)pollution of ground water
A)hurts human health
B)damages buildings and other human made structures
C)pollution of surface water
D)destroys forests
E)pollution of ground water

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
A solution which resists pH changes is
A)a hydrophilic solution.
B)a base.
C)a buffer.
D)a hydrophobic solution.
E)an acid.
A)a hydrophilic solution.
B)a base.
C)a buffer.
D)a hydrophobic solution.
E)an acid.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
A chemistry student measures the pH of a solution as 7.1.The student adds five milliliters of an acid to the solution and finds that the pH of the solution is still 7.1.What conclusion could be made from these observations?
A)The original solution was weakly basic.
B)The original solution was weakly acidic.
C)The original solution was strongly basiC.
D)The original solution was buffereD.
E)The original solution was strongly acidic.
A)The original solution was weakly basic.
B)The original solution was weakly acidic.
C)The original solution was strongly basiC.
D)The original solution was buffereD.
E)The original solution was strongly acidic.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
The attraction of an atom for the electrons in a covalent bond is called electronegativity.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
Radioactive isotopes can be used as tracers to detect molecular changes.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
Out of covalent,ionic,and hydrogen,the hydrogen bond is the strongest.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
Neutrons have a negative charge.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
What type of solution has a pH of 8.2?
A)neutral
B)acid
C)ionic
D)buffer
E)base
A)neutral
B)acid
C)ionic
D)buffer
E)base

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


