Deck 4: Energy and Physical Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 4: Energy and Physical Properties
1
Consider a bowling ball with the same diameter and volume as a soccer ball. Both are sitting still on the top of table. Both have the same potential energy.
False
2
Which of the following increases as temperature increases?
A)kinetic energy
B)attractive forces
C)potential energy
D)none of the above
A)kinetic energy
B)attractive forces
C)potential energy
D)none of the above
kinetic energy
3
Which of the following is the smallest unit of thermal energy?
A)calorie
B)Calorie
C)joule
D)kilojoule
A)calorie
B)Calorie
C)joule
D)kilojoule
joule
4
Specific heat is the conversion factor that allows for the calculation of energy based on temperature changes.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
The following is a mathematical expression of Guy-Lussac's law. 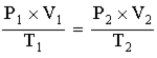
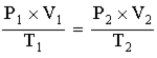

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
The specific heat of ice is 0.480 cal/g°C. How much heat will it take to raise the temperature of 22.50 g of ice from -30.0 °C to -20.0°C?
A)108 cal
B)216 cal
C)324 cal
D)469 cal
A)108 cal
B)216 cal
C)324 cal
D)469 cal

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
Either the Kelvin or the Celsius temperature scale can be used in gas law calculations.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
In order to convert a pressure in atmospheres to torr, the following conversion factor should be used. 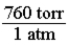
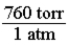

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Below is the structure of the amino acid, serine. Four hydrogen atoms in the compound can form hydrogen bonds. 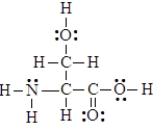


Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
Consider two identical soccer balls. Ball A is kicked and attains a velocity 25 m/sec. Ball B is kicked and attains a velocity of 15 m/sec. At these velocities, Ball A has the greater kinetic energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
The law of conservation of energy states which of the following?
A)Energy cannot be converted from one form to another.
B)Kinetic energy is conserved.
C)Potential energy is conserved.
D)none of the above
A)Energy cannot be converted from one form to another.
B)Kinetic energy is conserved.
C)Potential energy is conserved.
D)none of the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Butane is a gas at room temperature (25 °C). The boiling point of butane is probably greater than 25 °C.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
The two compounds below have the same molecular formula. One would expect A to stronger dispersion forces than B. 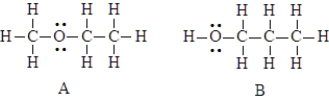
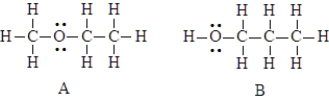

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
The two compounds below have the same molecular formula. One would expect A to have a higher boiling point than B. 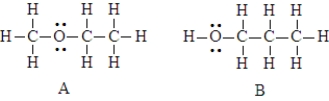
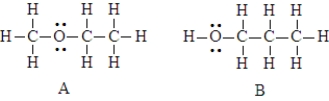

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
The pressure of a gas is dependent on temperature.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
Oxygen, nitrogen and helium are present in a container in which the total pressure is 735 torr. If the pressure of oxygen is 135 torr, that of helium is 55 torr, the pressure of nitrogen is 545 torr.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
To convert from joules to kilojoules, move the decimal in the number three places to the right.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
One would expect a compound with the molecular formula C4H10 to have a lower boiling point than one with the formula C7H16.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is true as a student slides down a waterslide?
A)Their kinetic energy decreases.
B)Their potential energy increases.
C)Their total energy increases.
D)none of the above
A)Their kinetic energy decreases.
B)Their potential energy increases.
C)Their total energy increases.
D)none of the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
Diethyl ether historically was used as an anesthetic. Currently, it is a very important solvent. The freezing point and boiling point of diethyl ether are -116 °C and 37 °C, respectively. Diethyl ether is a solid at 0 °C.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
Ethylene dichloride is an effective cooling agent when allowed to evaporate. The heat of vaporization is 85.3 cal/g. How much heat could be removed from the skin if 4.25g of ethylene dichloride were sprayed on and allowed to evaporate?
A)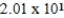 cal
cal
B)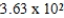 cal
cal
C)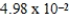 cal
cal
D)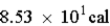
A)
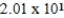 cal
calB)
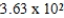 cal
calC)
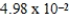 cal
calD)
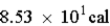

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
When alcohol is mixed with water which is the solvent?
A)the alcohol
B)the water
C)The substance present in larger amount.
D)The substance present in smaller amount.
A)the alcohol
B)the water
C)The substance present in larger amount.
D)The substance present in smaller amount.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
The heat of vaporization of water is 540 cal/g. What mass of water at 100.0 °C can be vaporized by the addition of 55.0 kcal of heat?
A)0.19 g
B)55 g
C)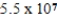 g
g
D)102 g
A)0.19 g
B)55 g
C)
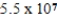 g
gD)102 g

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
In which of the following processes does the energy of the water increase?
A)water freezes
B)steam condenses to the liquid
C)water evaporates
D)water sublimes
E)both c and d
A)water freezes
B)steam condenses to the liquid
C)water evaporates
D)water sublimes
E)both c and d

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following best describes the relationship of molecules in a liquid with those in a gas at the same temperature?
A)Molecules in the liquid are much closer together than those in the gas.
B)Molecules in the liquid have less kinetic energy than those in the gas.
C)Molecules in the liquid are moving faster than those in the gas on the average.
D)Both a and b are correct.
E)All of the above are correct.
A)Molecules in the liquid are much closer together than those in the gas.
B)Molecules in the liquid have less kinetic energy than those in the gas.
C)Molecules in the liquid are moving faster than those in the gas on the average.
D)Both a and b are correct.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
Glycerol changes its shape to conform to its container and has a fairly high density. Glycerol is a:
A)solid.
B)liquid.
C)gaseous.
D)either b or c
A)solid.
B)liquid.
C)gaseous.
D)either b or c

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
Examine the following representation of a change of state. 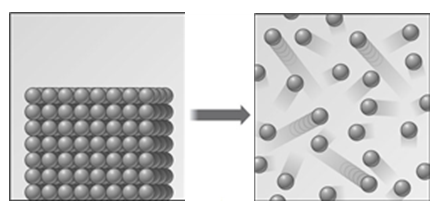
Which of the following is the correct term for this change?
A)freezing
B)sublimation
C)evaporation
D)boiling
E)melting

Which of the following is the correct term for this change?
A)freezing
B)sublimation
C)evaporation
D)boiling
E)melting

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following phase changes does not involve a solid?
A)evaporation
B)melting
C)sublimation
D)freezing
A)evaporation
B)melting
C)sublimation
D)freezing

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
Convert 0.662 atm to millibars.
A)377 millibar
B)671 millibar
C)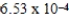 millibar
millibar
D)0.497 millibar
A)377 millibar
B)671 millibar
C)
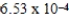 millibar
millibarD)0.497 millibar

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following molecules cannot engage in hydrogen bonding?
A)CH4
B)NH3
C)H2O
D)All of them can hydrogen bond.
A)CH4
B)NH3
C)H2O
D)All of them can hydrogen bond.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a type of heterogeneous mixture?
A)suspension
B)solution
C)colloid
D)solvent
A)suspension
B)solution
C)colloid
D)solvent

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
Air pressure can be expressed in which of the following units?
A)atmosphere
B)pascal
C)torr
D)any of these
A)atmosphere
B)pascal
C)torr
D)any of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
In which state of matter are the attractive forces between molecules the weakest?
A)solid
B)liquid
C)gas
D)None, the attractive forces are about the same in all.
A)solid
B)liquid
C)gas
D)None, the attractive forces are about the same in all.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the strongest intermolecular attractive force?
A)ion-ion attractions
B)dipole-dipole attractions
C)dispersion forces
D)hydrogen bonds
A)ion-ion attractions
B)dipole-dipole attractions
C)dispersion forces
D)hydrogen bonds

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following correctly arranges the physical states of matter (gas - g, solid - s, liquid - l) in order of increasing energy of the particles (lowest to highest)?
A)g < l < s
B)g < s < l
C)l < g < s
D)s < l < g
A)g < l < s
B)g < s < l
C)l < g < s
D)s < l < g

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
On a stove we have two pots of water at the boiling point. Pot 1 contains 1 L of water and pot 2 contains 2 L of water. Which of the following statements is true?
A)Pot 2 is at a higher temperature than pot 1.
B)Pot 2 has a larger thermal energy content than pot 1.
C)Both have the same thermal energy content.
D)Both a are b are true.
A)Pot 2 is at a higher temperature than pot 1.
B)Pot 2 has a larger thermal energy content than pot 1.
C)Both have the same thermal energy content.
D)Both a are b are true.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following molecules is most likely to be a gas at room temperature?
A)PH3
B)PCl3
C)PBr3
D)PI3
A)PH3
B)PCl3
C)PBr3
D)PI3

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following does not affect the boiling point of a liquid?
A)the formula weight of the liquid molecules
B)the shape of the liquid molecules
C)the intermolecular forces between the liquid molecules
D)All of the above affect the boiling point.
A)the formula weight of the liquid molecules
B)the shape of the liquid molecules
C)the intermolecular forces between the liquid molecules
D)All of the above affect the boiling point.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
At high altitudes atmospheric pressure is lower than at sea level. Which of the following is true?
A)Boiling water is cooler in Denver than in New York.
B)Boiling water is hotter in Denver than in New York.
C)Water boils at the same temperature in Denver and New York.
D)We cannot predict the relationship between the boiling points in the two cities.
A)Boiling water is cooler in Denver than in New York.
B)Boiling water is hotter in Denver than in New York.
C)Water boils at the same temperature in Denver and New York.
D)We cannot predict the relationship between the boiling points in the two cities.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
Potassium phosphate, K3PO4, is a solid at room temperature and is soluble in water. Which of the following statements is correct?
A)K3PO4 is an electrolyte.
B)K+ and PO43- ions are solvated by water.
C)K3PO4 completely dissociates in water.
D)All of the above are correct.
A)K3PO4 is an electrolyte.
B)K+ and PO43- ions are solvated by water.
C)K3PO4 completely dissociates in water.
D)All of the above are correct.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
In the following sentences,fill in the blanks with the appropriate terms from the list below. 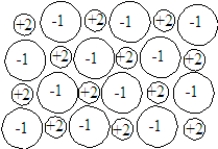
high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
This substance is water-soluble. A solution of this compound in water would be classified as a(n)_______________________.

high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
This substance is water-soluble. A solution of this compound in water would be classified as a(n)_______________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
In the following sentences,fill in the blanks with the appropriate terms from the list below. 
high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
The melting point of a solid composed of the particles shown in the image will be _________________.

high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
The melting point of a solid composed of the particles shown in the image will be _________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
A balloon with a volume of 5.65 L at room temperature (25°C) is placed in insulated container that is cooled with dry ice where the temperature is -19°C. What is the volume of the balloon in the container when its temperature reaches -19°C? Assume no change in pressure.
A)6.63 L
B)4.29 L
C)7.43 L
D)4.82 L
A)6.63 L
B)4.29 L
C)7.43 L
D)4.82 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
A metal gas cylinder is filled with compressed helium at a pressure 2.50 atm when the temperature is 37°C. What is the temperature in Celsius degrees when the pressure in the cylinder changes to 1.78 atm?
A)162 °C
B)-52 °C
C)221 °C
D)26 °C
A)162 °C
B)-52 °C
C)221 °C
D)26 °C

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
Helium (He)is used to fill balloons and is stored in a metal cylinder with pressure gauges on the top. 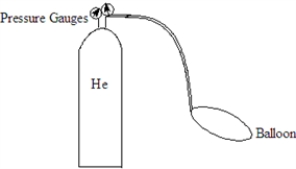 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
The cylinder was moved from the basement of the building to the fourth floor. The pressure reading _______________________.
 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.The cylinder was moved from the basement of the building to the fourth floor. The pressure reading _______________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
Helium (He)is used to fill balloons and is stored in a metal cylinder with pressure gauges on the top.  For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
The 175 g of He was added to the cylinder. The pressure reading ______________________.
 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.The 175 g of He was added to the cylinder. The pressure reading ______________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
Convert 0.917 atm to millibars.
A)697 millibars
B)929 millibars
C)523 millibars
D)1.223 millibars
A)697 millibars
B)929 millibars
C)523 millibars
D)1.223 millibars

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Helium (He)is used to fill balloons and is stored in a metal cylinder with pressure gauges on the top.  For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
Four balloons are filled from the cylinder. The pressure reading _______________________.
 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.Four balloons are filled from the cylinder. The pressure reading _______________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
Filling the blank with the appropriate term from the list below.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
A sample of helium gas is 1.00 atm. If you change the temperature of the sample from 40°C to 110°C and decrease the volume of the container, the resulting pressure can be calculated using ____________________ law.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
A sample of helium gas is 1.00 atm. If you change the temperature of the sample from 40°C to 110°C and decrease the volume of the container, the resulting pressure can be calculated using ____________________ law.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
A sample of nitrogen occupies a volume of 543 mL at 30°C and 772 torr. If the temperature is constant, what will be the pressure if the volume changes to 375 mL?
A)533 torr
B)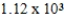 torr
torr
C)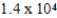 torr
torr
D)375 torr
A)533 torr
B)
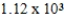 torr
torrC)
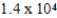 torr
torrD)375 torr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
In the following sentences,fill in the blanks with the appropriate terms from the list below. 
high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
_______________________is the type of force present between the particles shown in the image.

high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
_______________________is the type of force present between the particles shown in the image.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
Filling the blank with the appropriate term from the list below.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The effect of increasing the temperature on the volume of a gas can be calculated by using _________law.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The effect of increasing the temperature on the volume of a gas can be calculated by using _________law.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
The boiling point of the substance shown below will be ______________________ when compared to that of substance in the image.  .
.
 .
.
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Filling the blank with the appropriate term from the list below.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The variation in pressure with temperature is defined by ____________________law.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The variation in pressure with temperature is defined by ____________________law.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
An expandable plastic container contains 255 mL of nitrogen gas at 25°C and 688 torr. What is the volume of the container at 47°C and 489 torr?
A)385 mL
B)169 mL
C)195 mL
D)334 mL
E)341 mL
A)385 mL
B)169 mL
C)195 mL
D)334 mL
E)341 mL

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
Helium (He)is used to fill balloons and is stored in a metal cylinder with pressure gauges on the top.  For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
Sun shines on the cylinder and warms it, the pressure reading________________________.
 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.Sun shines on the cylinder and warms it, the pressure reading________________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
Air pressure can be expressed in which of the following units?
A)atmosphere
B)pascal
C)torr
D)any of these
A)atmosphere
B)pascal
C)torr
D)any of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
Filling the blank with the appropriate term from the list below.
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The following equation is an expression of ______________ law.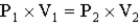
Charles's
Boyle's
Guy-Lussac's
Combined Gas
The following equation is an expression of ______________ law.
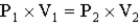

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
The use of the energy in food by the body to cause a muscle to contract is an example of
A)conversion of heat energy into potential energy.
B)conversion of kinetic energy into potential energy.
C)conversion of potential energy into kinetic energy.
D)conversion of kinetic energy into heal energy.
A)conversion of heat energy into potential energy.
B)conversion of kinetic energy into potential energy.
C)conversion of potential energy into kinetic energy.
D)conversion of kinetic energy into heal energy.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
Helium (He)is used to fill balloons and is stored in a metal cylinder with pressure gauges on the top.  For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
The cylinder was not secured and fell over, producing a sizable dent in the side. The pressure reading ____________________.
 For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.
For each of the following changes,fill the blank with one of the following terms to describe what happens to the pressure reading on the gauge.The cylinder was not secured and fell over, producing a sizable dent in the side. The pressure reading ____________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
When you inhale and exhale does your rib cage raise or lower? Why?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the image given below of a pair of ammonia molecules.Complete the following sentences using the appropriate terms listed below. 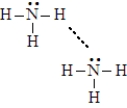
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The lower middle hydrogen atom in the upper left ammonia molecule could be a hydrogen bond _____________________.
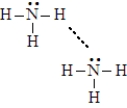
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The lower middle hydrogen atom in the upper left ammonia molecule could be a hydrogen bond _____________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following three substances. 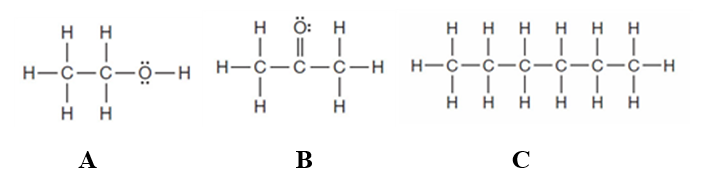
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
The substance capable of hydrogen bonding is _____.

Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
The substance capable of hydrogen bonding is _____.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following three substances. 
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance____can function as a hydrogen bond acceptor but not a hydrogen bond donor.

Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance____can function as a hydrogen bond acceptor but not a hydrogen bond donor.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the image given below of a pair of ammonia molecules.Complete the following sentences using the appropriate terms listed below. 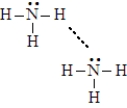
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The dashed line in the figure represents a__________________.
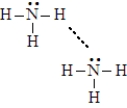
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The dashed line in the figure represents a__________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
What is the specific heat of a substance if it requires 475 cal to raise the temperature of a 67-gram sample from 25°C to 43°C?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the image given below of a pair of ammonia molecules.Complete the following sentences using the appropriate terms listed below. 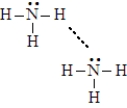
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
Each solid line in the figure represents a_______________________.
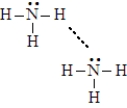
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
Each solid line in the figure represents a_______________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the image given below of a pair of ammonia molecules.Complete the following sentences using the appropriate terms listed below. 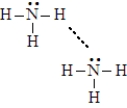
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The nitrogen atom in the lower right ammonia molecule is a hydrogen bond _____________________.
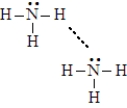
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
The nitrogen atom in the lower right ammonia molecule is a hydrogen bond _____________________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
The volume of a gas in the upper atmosphere needs to be calculated. If the temperature is -41°C, what numerical value should be used in the calculation?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following three substances. 
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance _____ would form a heterogeneous mixture with water.

Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance _____ would form a heterogeneous mixture with water.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
A gas cylinder contains three gases:
oxygen, nitrogen, and helium. The partial pressure of oxygen is 4.7 psi and the partial pressure of nitrogen is 3.0 psi. If the reading on the pressure gauge is as shown below. What is the partial pressure of the He?
What is the partial pressure of the He?
oxygen, nitrogen, and helium. The partial pressure of oxygen is 4.7 psi and the partial pressure of nitrogen is 3.0 psi. If the reading on the pressure gauge is as shown below.
 What is the partial pressure of the He?
What is the partial pressure of the He?
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following three substances. 
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance(s) that _____ is(are) soluble in water.

Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
Substance(s) that _____ is(are) soluble in water.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
A convenient reference set of conditions for gases is STP (Standard Temperature and Pressure). There conditions are defined as exactly 0°C and 1.00 atm. If a sample of a gas have a volume of 2.57 L at a temperature of 27°C and 1.25 atm. What is the volume of this gas at STP?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


