Exam 4: Energy and Physical Properties
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
In the following sentences,fill in the blanks with the appropriate terms from the list below. 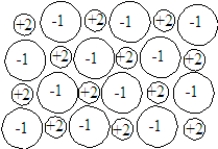 high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
-The melting point of a solid composed of the particles shown in the image will be _________________.
high
low
electrolyte
nonelectrolyte
ion-ion attraction
dipole-dipole attraction
hydrogen bond
dispersion force
-The melting point of a solid composed of the particles shown in the image will be _________________.
Free
(Short Answer)
5.0/5  (36)
(36)
Correct Answer:
high
High
Ethylene dichloride is an effective cooling agent when allowed to evaporate. The heat of vaporization is 85.3 cal/g. How much heat could be removed from the skin if 4.25g of ethylene dichloride were sprayed on and allowed to evaporate?
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
B
Which of the following increases as temperature increases?
Free
(Multiple Choice)
4.9/5  (21)
(21)
Correct Answer:
A
A convenient reference set of conditions for gases is STP (Standard Temperature and Pressure). There conditions are defined as exactly 0°C and 1.00 atm. If a sample of a gas have a volume of 2.57 L at a temperature of 27°C and 1.25 atm. What is the volume of this gas at STP?
(Short Answer)
4.7/5  (38)
(38)
In which state of matter are the attractive forces between molecules the weakest?
(Multiple Choice)
4.7/5  (30)
(30)
Specific heat is the conversion factor that allows for the calculation of energy based on temperature changes.
(True/False)
4.9/5  (38)
(38)
Consider the following three substances. 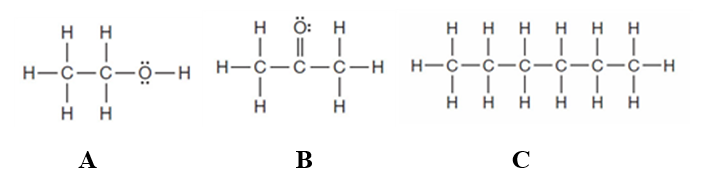 Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
-Substance(s) that _____ is(are) soluble in water.
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
-Substance(s) that _____ is(are) soluble in water.
(Short Answer)
4.9/5  (33)
(33)
Consider the image given below of a pair of ammonia molecules.Complete the following sentences using the appropriate terms listed below. 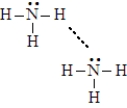 covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
-The dashed line in the figure represents a__________________.
covalent bond
hydrogen bond
ion-dipole attraction
donor
acceptor
-The dashed line in the figure represents a__________________.
(Short Answer)
4.9/5  (33)
(33)
To convert from joules to kilojoules, move the decimal in the number three places to the right.
(True/False)
4.8/5  (37)
(37)
At high altitudes atmospheric pressure is lower than at sea level. Which of the following is true?
(Multiple Choice)
4.9/5  (29)
(29)
Air pressure can be expressed in which of the following units?
(Multiple Choice)
4.9/5  (34)
(34)
The two compounds below have the same molecular formula. One would expect A to stronger dispersion forces than B. 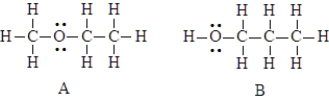
(True/False)
4.7/5  (37)
(37)
A gas cylinder contains three gases:
oxygen, nitrogen, and helium. The partial pressure of oxygen is 4.7 psi and the partial pressure of nitrogen is 3.0 psi. If the reading on the pressure gauge is as shown below.  What is the partial pressure of the He?
What is the partial pressure of the He?
(Short Answer)
5.0/5  (34)
(34)
Air pressure can be expressed in which of the following units?
(Multiple Choice)
4.8/5  (33)
(33)
One would expect a compound with the molecular formula C4H10 to have a lower boiling point than one with the formula C7H16.
(True/False)
4.8/5  (35)
(35)
Which of the following is true as a student slides down a waterslide?
(Multiple Choice)
4.7/5  (34)
(34)
An expandable plastic container contains 255 mL of nitrogen gas at 25°C and 688 torr. What is the volume of the container at 47°C and 489 torr?
(Multiple Choice)
4.8/5  (28)
(28)
Consider the following three substances.  Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
-Substance _____ would form a heterogeneous mixture with water.
Enter the letter of the appropriate substance in the blank provided.If none applies,enter the word none.
-Substance _____ would form a heterogeneous mixture with water.
(Short Answer)
4.8/5  (33)
(33)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)