Deck 2: Particles of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 2: Particles of Matter
1
You take 50 mL of small BB's and combine them with 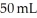 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
A)Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B)This is not possible since the Law of Conservation of Volume would be violated.
C)The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D)Many of the smaller BB's are able to fit within the pockets of space that were empty within the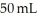 of large BB's.
of large BB's.
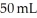 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?A)Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B)This is not possible since the Law of Conservation of Volume would be violated.
C)The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D)Many of the smaller BB's are able to fit within the pockets of space that were empty within the
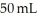 of large BB's.
of large BB's.Many of the smaller BB's are able to fit within the pockets of space that were empty within the 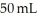 of large BB's.
of large BB's.
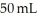 of large BB's.
of large BB's. 2
Aristotle described the composition and behavior of matter ________.
A)in terms of the 4 qualities of hot, cold, moist and dry
B)as the elements of fire, water, air and earth
C)as materials that are unchanging
D)as states of perception
A)in terms of the 4 qualities of hot, cold, moist and dry
B)as the elements of fire, water, air and earth
C)as materials that are unchanging
D)as states of perception
in terms of the 4 qualities of hot, cold, moist and dry
3
A TV screen looked at from a distance appears as a smooth continuous flow of images. Up close, however, we see this is an illusion. What really exists are a series of tiny dots (pixels). This is similar to a chemist's view of matter in that ________.
A)the fundamental particles of matter can also be seen when looked at closely with a magnifying glass
B)on the submicroscopic level, chemist's find that matter is made of extremely small particles, such as atoms and molecules.
C)anything that a chemist can see, touch, hear, smell, or taste is an illusion
D)elements are made up of only three basic types of matter
A)the fundamental particles of matter can also be seen when looked at closely with a magnifying glass
B)on the submicroscopic level, chemist's find that matter is made of extremely small particles, such as atoms and molecules.
C)anything that a chemist can see, touch, hear, smell, or taste is an illusion
D)elements are made up of only three basic types of matter
on the submicroscopic level, chemist's find that matter is made of extremely small particles, such as atoms and molecules.
4
The same amount of red colored Kool-Aid crystals are added to a still glass of thick sugar water and a still glass of distilled water. Both are the same temperature. Neither is stirred. Which should become uniform in color first?
A)The glass of distilled water should become uniform in color first.
B)The glass of thick sugar water should become uniform in color first.
C)Both glasses will become uniform in color at exactly the same time.
D)Without stirring, it is unlikely that either glass will ever become uniform in color.
A)The glass of distilled water should become uniform in color first.
B)The glass of thick sugar water should become uniform in color first.
C)Both glasses will become uniform in color at exactly the same time.
D)Without stirring, it is unlikely that either glass will ever become uniform in color.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
Based on the Law of Mass Conservation, Lavoisier hypothesized that ________.
A)an element is made of a fundamental substance that cannot be broken down into anything else
B)matter can lose or gain mass as hot, dry, cold or moist qualities change
C)an element is a combination of substances
D)water is a fundamental element
A)an element is made of a fundamental substance that cannot be broken down into anything else
B)matter can lose or gain mass as hot, dry, cold or moist qualities change
C)an element is a combination of substances
D)water is a fundamental element

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
Based on experimental evidence, John Dalton postulated that ________.
A)each element consists of indivisible, minute particles called atoms
B)atoms can be created and destroyed in chemical reactions
C)not all atoms of a given element are identical
D)atoms of different elements have the same masses
A)each element consists of indivisible, minute particles called atoms
B)atoms can be created and destroyed in chemical reactions
C)not all atoms of a given element are identical
D)atoms of different elements have the same masses

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
Dalton's atomic model gained credibility because ________.
A)atoms are too small to be seen, so no one could prove him wrong
B)the model worked to explain much about known chemical reactions
C)people could make gold from common metals
D)he was elected into the Royal Society
A)atoms are too small to be seen, so no one could prove him wrong
B)the model worked to explain much about known chemical reactions
C)people could make gold from common metals
D)he was elected into the Royal Society

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
According to Aristotle's hypothesis about matter, wet clay is converted to ceramic because ________.
A)heat drives out the coolness of earth
B)dry air replaces moist air
C)fire replaces the moist element with the dry element
D)dry quality is replaced with moist quality
A)heat drives out the coolness of earth
B)dry air replaces moist air
C)fire replaces the moist element with the dry element
D)dry quality is replaced with moist quality

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
A biological cell is best described as ________.
A)macroscopic
B)microscopic
C)submicroscopic
D)nanoscopic
A)macroscopic
B)microscopic
C)submicroscopic
D)nanoscopic

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
In what sense can you truthfully say that you are a part of every person around you?
A)We all live on the same planet and share the same resources.
B)We are continually exchanging our atoms.
C)We all share the same genetic code.
D)There are more people alive now than have ever lived.
A)We all live on the same planet and share the same resources.
B)We are continually exchanging our atoms.
C)We all share the same genetic code.
D)There are more people alive now than have ever lived.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
Dmitri Mendeleev's chart of elements ________.
A)was used as a calendar
B)placed elements together with similar properties
C)shifted the elements to fill in the gaps
D)had many defects because of unknown elements
A)was used as a calendar
B)placed elements together with similar properties
C)shifted the elements to fill in the gaps
D)had many defects because of unknown elements

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
Considering how small atoms are, what are the chances that at least one of the atoms exhaled in your first breath will be in your last breath?
A)not very likely because atoms are constantly dematerializing
B)not possible at all because atom don't last that long
C)very probable because of how incredibly small atoms are
D)There's not really a way that scientists are able to make such an estimate.
A)not very likely because atoms are constantly dematerializing
B)not possible at all because atom don't last that long
C)very probable because of how incredibly small atoms are
D)There's not really a way that scientists are able to make such an estimate.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
Dmitri Mendeleev ________.
A)predicted the existence of elements not yet discovered
B)developed the basis of our modern periodic table
C)helped in the discovery of missing elements by predicting their behavior
D)all of the above
A)predicted the existence of elements not yet discovered
B)developed the basis of our modern periodic table
C)helped in the discovery of missing elements by predicting their behavior
D)all of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
How would you describe the size of the following object? a blood cell
A)microscopic
B)macroscopic
C)submicroscopic
D)all of the above
E)none of the above
A)microscopic
B)macroscopic
C)submicroscopic
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
According to John Dalton, atoms of a given element ________.
A)are identical
B)have different masses
C)are divisible
D)have the same shape
A)are identical
B)have different masses
C)are divisible
D)have the same shape

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
How does Aristotle's model of matter explain how a puddle of water disappears to dryness on a sunny day?
A)Aristotle's model explains that water becomes dry when it comes into contact with Earth.
B)Aristotle's model explains that moisture combines with heat to form air.
C)Aristotle's model explains that the sun directs Earth to absorb the puddle of water.
D)Aristotle's model has no explanation of how a puddle of water disappears to dryness on a sunny day.
A)Aristotle's model explains that water becomes dry when it comes into contact with Earth.
B)Aristotle's model explains that moisture combines with heat to form air.
C)Aristotle's model explains that the sun directs Earth to absorb the puddle of water.
D)Aristotle's model has no explanation of how a puddle of water disappears to dryness on a sunny day.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
How would you describe the volume of the following object? the amount of water in a swimming pool
A)microscopic
B)macroscopic
C)submicroscopic
D)all of the above
E)none of the above
A)microscopic
B)macroscopic
C)submicroscopic
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
How does Aristotle's model of matter explain why the air over a flame is always moist?
A)Aristotle's model of matter explains that moisture in the air draws heat out of the flame.
B)Aristotle's model of matter explains that moisture counters heat since water counters fire.
C)Aristotle's model of matter would explain quite the opposite and predict the air above the flame would become dry.
D)Aristotle's model does not deal with the concept of air over a flame.
A)Aristotle's model of matter explains that moisture in the air draws heat out of the flame.
B)Aristotle's model of matter explains that moisture counters heat since water counters fire.
C)Aristotle's model of matter would explain quite the opposite and predict the air above the flame would become dry.
D)Aristotle's model does not deal with the concept of air over a flame.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
Alchemist of the Middle Ages believed that ________.
A)atoms are the basis of matter
B)materials can be altered to produce new molecules
C)a substance can be transformed into gold by altering its basic qualities
D)iron is strong and springy because its atoms are shaped like coils
A)atoms are the basis of matter
B)materials can be altered to produce new molecules
C)a substance can be transformed into gold by altering its basic qualities
D)iron is strong and springy because its atoms are shaped like coils

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
Red colored Kool-Aid crystals are added to a still glass of hot water. The same amount of crystals are added to a second still glass filled with the same amount of cold water. With no stirring, which of the following would occur?
A)Without stirring, both glasses will reach uniform color in the same amount of time since they both contain identical amounts of water.
B)The glass of cold water will reach a uniform red color first since there are no heat convection currents to impede the distribution of the dye.
C)The glass of hot water will reach a uniform red color first since the higher kinetic energy provides for faster moving molecules to more quickly distribute the dye.
D)The Kool-aid crystals will never dissolve in either glass until the glasses are stirred.
A)Without stirring, both glasses will reach uniform color in the same amount of time since they both contain identical amounts of water.
B)The glass of cold water will reach a uniform red color first since there are no heat convection currents to impede the distribution of the dye.
C)The glass of hot water will reach a uniform red color first since the higher kinetic energy provides for faster moving molecules to more quickly distribute the dye.
D)The Kool-aid crystals will never dissolve in either glass until the glasses are stirred.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
A little girl sits in a car at a traffic light holding a helium-filled balloon as shown in the illustration below. The windows are closed and the car is relatively airtight. When the light turns green and the car accelerates forward, her head pitches backward but the balloon pitches forward. Why? 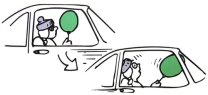
A)This demonstration will only work with a helium filled mylar balloon since the mylar is unaffected by the motion of the air moving backwards inside the car.
B)Since helium is lighter than air, it will follow the path of the car and not the direction of movement of the air within it.
C)Since the car is relatively airtight and the balloon is not anchored to the car, the balloon will be unaffected by the acceleration of the car.
D)The air inside the car has more inertia than the helium in the helium balloon. Therefore, although the air pitches backwards, the lighter helium moves forward.

A)This demonstration will only work with a helium filled mylar balloon since the mylar is unaffected by the motion of the air moving backwards inside the car.
B)Since helium is lighter than air, it will follow the path of the car and not the direction of movement of the air within it.
C)Since the car is relatively airtight and the balloon is not anchored to the car, the balloon will be unaffected by the acceleration of the car.
D)The air inside the car has more inertia than the helium in the helium balloon. Therefore, although the air pitches backwards, the lighter helium moves forward.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
Gravity on the moon is only 1/6 as strong as gravity on the earth. What is the mass of a 10 kg object on the moon and on the earth?
A)A 10 kg object weighs 10 kg on Earth and 10/6 = 1.67 kg on the moon.
B)The mass of an object is indirectly proportional to the mass of the planet on which it is located. Therefore, a 10 kg object weighs 10/6 = 1.67 kg on Earth and 10 kg on the moon.
C)The mass of a 10 kg object anywhere is 10 kg.
D)The mass of a 10 kg object on Earth is 22 pounds, while on the moon it is 3.7 pounds.
E)The 10 kg object weighs 60 kg on the moon.
A)A 10 kg object weighs 10 kg on Earth and 10/6 = 1.67 kg on the moon.
B)The mass of an object is indirectly proportional to the mass of the planet on which it is located. Therefore, a 10 kg object weighs 10/6 = 1.67 kg on Earth and 10 kg on the moon.
C)The mass of a 10 kg object anywhere is 10 kg.
D)The mass of a 10 kg object on Earth is 22 pounds, while on the moon it is 3.7 pounds.
E)The 10 kg object weighs 60 kg on the moon.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following does not describe mass?
A)how much space an object occupies
B)kilograms
C)how much matter is in a given object
D)the amount of inertia in a given object
E)grams
A)how much space an object occupies
B)kilograms
C)how much matter is in a given object
D)the amount of inertia in a given object
E)grams

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
Which would you rather have: a decigram or a kilogram of gold?
A)a decigram of gold because this is 100 times as much as a kilogram of gold
B)a decigram of gold because this is 1000 times as much as a kilogram of gold
C)a kilogram of gold because this is 1000 times as much as a decigram of gold
D)a kilogram of gold because this is 10,000 times as much as a decigram of gold
A)a decigram of gold because this is 100 times as much as a kilogram of gold
B)a decigram of gold because this is 1000 times as much as a kilogram of gold
C)a kilogram of gold because this is 1000 times as much as a decigram of gold
D)a kilogram of gold because this is 10,000 times as much as a decigram of gold

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
Your weight is always ________.
A)greater than your mass
B)equal to your mass
C)less than your mass
D)not the same thing as your mass
A)greater than your mass
B)equal to your mass
C)less than your mass
D)not the same thing as your mass

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the largest quantity of mass?
A)100 kg
B)100,000 g
C)100,000,000 mg
D)All of the masses are the same.
A)100 kg
B)100,000 g
C)100,000,000 mg
D)All of the masses are the same.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
Does a 2 kg solid iron brick have twice as much mass as a 1 kg solid block of wood? Twice as much volume?
A)The iron brick has twice the mass as well as twice the volume.
B)The iron brick has twice the mass, but only half the volume.
C)The iron brick has twice the mass, but its volume compared to the block of wood depends on the density of the wood.
D)The iron brick has twice the mass, but its volume compared to the block of wood depends on the weight of the wood.
A)The iron brick has twice the mass as well as twice the volume.
B)The iron brick has twice the mass, but only half the volume.
C)The iron brick has twice the mass, but its volume compared to the block of wood depends on the density of the wood.
D)The iron brick has twice the mass, but its volume compared to the block of wood depends on the weight of the wood.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is not a measurement of volume?
A)0.156 liter
B)22.02 milliliters
C)10.0 cubic centimeters
D)842 cubic meters
E)5.5 milligrams
A)0.156 liter
B)22.02 milliliters
C)10.0 cubic centimeters
D)842 cubic meters
E)5.5 milligrams

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is not a volume measurement?
A)0.156 liter
B)22.02 milliliters
C)10.0 cubic centimeters
D)842 cubic meters
E)5.5 milligrams
A)0.156 liter
B)22.02 milliliters
C)10.0 cubic centimeters
D)842 cubic meters
E)5.5 milligrams

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
What is the mass in kilograms of a 130-pound human standing on planet Earth?
A)about 290 kg
B)about 59 kg
C)about 130 kg
D)about 22 kg
A)about 290 kg
B)about 59 kg
C)about 130 kg
D)about 22 kg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
The scanning probe microscope creates images of atoms by ________.
A)passing an electric current between the tip of an ultrathin needle and some conducting surface
B)measuring the up and down motions of an ultrathin needle
C)processing a series of numbers into a computer generated image of atoms
D)all of the above
A)passing an electric current between the tip of an ultrathin needle and some conducting surface
B)measuring the up and down motions of an ultrathin needle
C)processing a series of numbers into a computer generated image of atoms
D)all of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
What physical quantities discussed in this chapter change most when a junked car is neatly crushed into a compact cube?
A)The car's weight and volume change, but not its mass.
B)The volume of the car changes as well as its average density.
C)The car's temperature changes, but not its average density.
D)The mass of the car changes as well as its volume.
A)The car's weight and volume change, but not its mass.
B)The volume of the car changes as well as its average density.
C)The car's temperature changes, but not its average density.
D)The mass of the car changes as well as its volume.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
Why is it not possible for the scanning probe microscope (SPM)to make images of the inside of an atom?
A)The most representative "image" of the inside of the atom would be a picture of empty space.
B)The SPM works by passing a current across a tiny gap between the tip of an ultrathin needle and the surface being studied. No gap, no image.
C)The SPM ultrathin needle itself is made of atoms and so is not tiny enough to pierce through an atom much like a sewing needle can pierce into a Ping-Pong ball.
D)All of the above are reasonable answers.
A)The most representative "image" of the inside of the atom would be a picture of empty space.
B)The SPM works by passing a current across a tiny gap between the tip of an ultrathin needle and the surface being studied. No gap, no image.
C)The SPM ultrathin needle itself is made of atoms and so is not tiny enough to pierce through an atom much like a sewing needle can pierce into a Ping-Pong ball.
D)All of the above are reasonable answers.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following does not describe volume?
A)the weight of a given object
B)liters
C)the amount of space a material occupies
D)a box 20 cm × 20 cm × 20 cm
E)milliliters
A)the weight of a given object
B)liters
C)the amount of space a material occupies
D)a box 20 cm × 20 cm × 20 cm
E)milliliters

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
When a concentrated acid and fresh water, both at room temperature, are mixed together the result is a solution that is very hot. How does Aristotle's model of matter explain this?
A)Aristotle's model says that two forms of "water" always combine to produce heat.
B)That two forms of "water" could combine to produce heat is counter to Aristotle's model of matter.
C)Aristotle's model says that heat is produced ONLY if the water is added to the acid.
D)Aristotle's model says that heat is produced ONLY if the acid is added to the water.
A)Aristotle's model says that two forms of "water" always combine to produce heat.
B)That two forms of "water" could combine to produce heat is counter to Aristotle's model of matter.
C)Aristotle's model says that heat is produced ONLY if the water is added to the acid.
D)Aristotle's model says that heat is produced ONLY if the acid is added to the water.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
Can an object have mass without having weight? Can it have weight without having mass?
A)yes and yes
B)yes and no
C)no and yes
D)no and no
A)yes and yes
B)yes and no
C)no and yes
D)no and no

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following represents the largest quantity of mass?
A)24 grams
B)24 milligrams
C)24 kilograms
D)24 micrograms
E)24 nanograms
A)24 grams
B)24 milligrams
C)24 kilograms
D)24 micrograms
E)24 nanograms

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
What is the difference between mass and weight?
A)Weight is the observed effect of gravity on mass.
B)Mass is how much; weight is how much space.
C)Weight is how much; mass is how much space.
D)Weight and mass are the same everywhere.
E)Mass is the observed effect of inertia on weight.
A)Weight is the observed effect of gravity on mass.
B)Mass is how much; weight is how much space.
C)Weight is how much; mass is how much space.
D)Weight and mass are the same everywhere.
E)Mass is the observed effect of inertia on weight.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
Why do we use different units for mass and weight?
A)They are two different quantities.
B)Mass is metric and weight is U.S Customary Standard.
C)Weight is metric and mass is U.S Customary Standard.
D)Weight is how heavy something is and mass is how much space it takes up.
E)Actually, mass and weight share the same units.
A)They are two different quantities.
B)Mass is metric and weight is U.S Customary Standard.
C)Weight is metric and mass is U.S Customary Standard.
D)Weight is how heavy something is and mass is how much space it takes up.
E)Actually, mass and weight share the same units.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
The gravity of the moon is 1/6 that of Earth's. If your mass is 65 kilograms on Earth, what is your mass on the moon?
A)65 kg
B)10.8 kg
C)390 kg
D)39.0 kg
E)6.5 kg
A)65 kg
B)10.8 kg
C)390 kg
D)39.0 kg
E)6.5 kg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
The following three boxes represent the number of submicroscopic particles within a given volume of a particular substance at different temperatures. Which box represents the greatest density? Which box represents the greatest temperature? 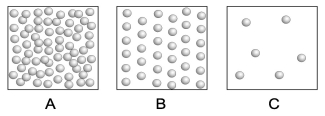
A)A and C
B)B and C
C)A and B
D)B and A

A)A and C
B)B and C
C)A and B
D)B and A

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
Assuming temperature remains constant, what happens to the density of a gas as the gas is compressed into a smaller volume?
A)The density of the gas decreases along with the decreasing volume.
B)The density of the gas increases along with the decreasing volume.
C)The density of the gas stays the same because it is an intrinsic property of a material.
D)The density increases because of an increase in mass.
A)The density of the gas decreases along with the decreasing volume.
B)The density of the gas increases along with the decreasing volume.
C)The density of the gas stays the same because it is an intrinsic property of a material.
D)The density increases because of an increase in mass.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
If the density of mercury is 13.6 g/mL and the density of lead is 11.3 g/mL, which has the larger volume: 1 g of mercury or 1 g of lead?
A)lead
B)mercury
C)Volume and mass are not related.
D)Both have the same volume.
E)none of the above
A)lead
B)mercury
C)Volume and mass are not related.
D)Both have the same volume.
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
How many milliliters of air are there in a hole measuring 5 L?
A)5000 mL
B)200 mL
C)5 mL
D)There are no milliliters of air in this hole.
A)5000 mL
B)200 mL
C)5 mL
D)There are no milliliters of air in this hole.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is an example of something with potential energy?
A)a boulder at the top of a cliff
B)an arrow poised in a stretched bow
C)chemical bonds
D)gasoline in the gas tank of your car
E)all of the above
A)a boulder at the top of a cliff
B)an arrow poised in a stretched bow
C)chemical bonds
D)gasoline in the gas tank of your car
E)all of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
When water freezes, it expands. What does this say about the density of ice compared with the density of water?
A)The density of water and ice are identical since they have exactly the same chemical formula.
B)The expansion of water when it freezes to ice changes its volume, not its density.
C)Ice is less dense than water because it has more volume for the same mass.
D)Ice is more dense than water because it has more volume for the same mass
A)The density of water and ice are identical since they have exactly the same chemical formula.
B)The expansion of water when it freezes to ice changes its volume, not its density.
C)Ice is less dense than water because it has more volume for the same mass.
D)Ice is more dense than water because it has more volume for the same mass

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
What is the mass in kilograms of a human weighing 130-pounds on the moon?
A)about 350 kg
B)about 48 kg
C)about 22 kg
D)about 130 kg
A)about 350 kg
B)about 48 kg
C)about 22 kg
D)about 130 kg

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is something that is best described as having potential energy?
A)food
B)wind
C)running water
D)a meteorite traveling through the atmosphere
E)none of the above
A)food
B)wind
C)running water
D)a meteorite traveling through the atmosphere
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
A post-1982 penny is made with zinc, but its density is actually greater than that of zinc because ________.
A)it is coated with a thin layer of copper, which is more dense than zinc
B)it is compressed to a smaller volume in the manufacturing process
C)it has more mass for the same volume
D)Both A and C are correct
A)it is coated with a thin layer of copper, which is more dense than zinc
B)it is compressed to a smaller volume in the manufacturing process
C)it has more mass for the same volume
D)Both A and C are correct

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
If the density of a block of ice is 0.92 g/mL, what is the volume of 100. g of ice?
A)109 mL
B)92.0 mL
C)0.920 mL
D)10.9 mL
E)0.109 mL
A)109 mL
B)92.0 mL
C)0.920 mL
D)10.9 mL
E)0.109 mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is generally true regarding the relationships of the density of an object and its massiveness?
A)A denser object is not necessarily always more massive.
B)There is no scientific relationship between density and massiveness.
C)The relationship between density and massiveness depends on the physical state of the object only.
D)None of the above is true.
A)A denser object is not necessarily always more massive.
B)There is no scientific relationship between density and massiveness.
C)The relationship between density and massiveness depends on the physical state of the object only.
D)None of the above is true.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
What happens to the density of a filled water balloon as it is pulled to the bottom of the ocean?
A)The density of the filled water balloon does not change. The increase in pressure is offset by a decrease in water temperature.
B)The density of the filled water balloon decreases as more cold water surrounds the balloon at greater depth.
C)The density of the filled water balloon increases since the balloon is compressed to a smaller volume.
D)The density of the filled water balloon first increases because of a decrease in water temperature, then the density decreases at greater depths as the pressure increases.
A)The density of the filled water balloon does not change. The increase in pressure is offset by a decrease in water temperature.
B)The density of the filled water balloon decreases as more cold water surrounds the balloon at greater depth.
C)The density of the filled water balloon increases since the balloon is compressed to a smaller volume.
D)The density of the filled water balloon first increases because of a decrease in water temperature, then the density decreases at greater depths as the pressure increases.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
Someone wants to sell you a piece of gold that they say is nearly pure. Before buying the piece, you measure its mass to be 52.3 grams and you find that it displaces 4.16 mL of water. Calculate its density and assess its purity given the fact that pure gold has a density of 19.3 g/mL.
A)The piece of gold is about 65 percent pure.
B)The piece of gold is only about 12.6 percent pure.
C)The piece of gold is only about 8 percent pure.
D)Not enough information is given to calculate the purity, but enough information is given to let you know that the piece is far from pure.
A)The piece of gold is about 65 percent pure.
B)The piece of gold is only about 12.6 percent pure.
C)The piece of gold is only about 8 percent pure.
D)Not enough information is given to calculate the purity, but enough information is given to let you know that the piece is far from pure.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is an example of something best described as having kinetic energy?
A)a ball falling through the air
B)a boulder at the bottom of a cliff
C)a dart stuck into a dart board
D)a candy bar with 230 kilocalories
E)a log of wood in a fireplace that has not been lit
A)a ball falling through the air
B)a boulder at the bottom of a cliff
C)a dart stuck into a dart board
D)a candy bar with 230 kilocalories
E)a log of wood in a fireplace that has not been lit

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following units of measurement could be used to describe density?
A)kilograms per liter
B)miles per hour
C)grams per kilogram
D)feet per gram
E)milliliters per gram
A)kilograms per liter
B)miles per hour
C)grams per kilogram
D)feet per gram
E)milliliters per gram

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following has the largest density?
A)a 10-g object with a volume of 1 mL
B)a 1-g object with a volume of 10 mL
C)a 200-g object with a volume of 200 mL
D)a 10-g object with a volume of 10 mL
E)All of the densities are the same.
A)a 10-g object with a volume of 1 mL
B)a 1-g object with a volume of 10 mL
C)a 200-g object with a volume of 200 mL
D)a 10-g object with a volume of 10 mL
E)All of the densities are the same.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
A block of wood that weighs 10. g is immersed in water. The total amount of water displaced is 12 mL. What is the density of the block of wood?
A)0.83 g/mL
B)1.2 g/mL
C)0.83 mL/g
D)12 g/10 mL
E)120 g/mL
A)0.83 g/mL
B)1.2 g/mL
C)0.83 mL/g
D)12 g/10 mL
E)120 g/mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
What volume of water would a 52.3-gram sample of pure gold displace? (Assume the density of pure gold equals 19.3 g/mL)
A)36.9 mL
B)2.71 mL
C)52.3 mL
D)The density of water should be given in order to make this calculation possible.
A)36.9 mL
B)2.71 mL
C)52.3 mL
D)The density of water should be given in order to make this calculation possible.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
With increasing temperature the density of air ________.
A)increases
B)decreases
C)stays the same
D)depends also on pressure
A)increases
B)decreases
C)stays the same
D)depends also on pressure

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
A piece of aluminum weighing 10.0 grams is placed into a graduated cylinder that has an initial volume (before immersion)of 35.0 mL. After immersion, the volume on the cylinder reads 38.7 mL. What is the density of the metal sample?
A)2.70 g/mL
B)0.25 g/mL
C)0.28 g/mL
D)0.37 g/mL
E)3.70 g/mL
A)2.70 g/mL
B)0.25 g/mL
C)0.28 g/mL
D)0.37 g/mL
E)3.70 g/mL

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
In which are the molecules moving faster: a swimming pool of boiling water or a cup of boiling water?
A)The swimming pool of boiling water because it contains more energy.
B)The cup because the molecules rebound off the container's inner surface more frequently.
C)The average motion of the molecules in each is the same.
D)There is insufficient information given to determine where the molecules move faster.
A)The swimming pool of boiling water because it contains more energy.
B)The cup because the molecules rebound off the container's inner surface more frequently.
C)The average motion of the molecules in each is the same.
D)There is insufficient information given to determine where the molecules move faster.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the least amount of energy?
A)1.0 joules
B)1.0 kilojoule
C)1.0 Calorie
D)1.0 kilocalorie
E)1.0 calorie
A)1.0 joules
B)1.0 kilojoule
C)1.0 Calorie
D)1.0 kilocalorie
E)1.0 calorie

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
Which is more evident: potential or kinetic energy?
A)Potential energy is more evident than kinetic energy because it required work in order to gain that potential energy.
B)Potential energy is more evident than kinetic energy because it involves motion.
C)Kinetic energy is more evident than potential energy because it is created from potential energy.
D)Kinetic energy is more evident than potential energy because it involves motion.
A)Potential energy is more evident than kinetic energy because it required work in order to gain that potential energy.
B)Potential energy is more evident than kinetic energy because it involves motion.
C)Kinetic energy is more evident than potential energy because it is created from potential energy.
D)Kinetic energy is more evident than potential energy because it involves motion.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following best describes temperature?
A)Temperature is the measure of the average amount of kinetic energy in a substance.
B)Temperature is the measure of the total amount of energy in a substance.
C)Temperature is the measure of the heat of an object.
D)all of the above
E)none of the above
A)Temperature is the measure of the average amount of kinetic energy in a substance.
B)Temperature is the measure of the total amount of energy in a substance.
C)Temperature is the measure of the heat of an object.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
If you drop a hot rock into a pail of water, the temperature of the rock and the water change until the two are equal. The rock cools and the water warms. Does this hold true if the hot rock is dropped into the Atlantic Ocean?
A)no, because the Atlantic Ocean is so much bigger than a pail of water
B)no, because the Atlantic Ocean also contains dissolved salts
C)yes, because heat will continue to flow until they have the same temperature
D)yes, because the Atlantic Ocean actually contains more energy than does the pail of water
A)no, because the Atlantic Ocean is so much bigger than a pail of water
B)no, because the Atlantic Ocean also contains dissolved salts
C)yes, because heat will continue to flow until they have the same temperature
D)yes, because the Atlantic Ocean actually contains more energy than does the pail of water

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
An old remedy for separating a pair of nested drinking glasses stuck together is to run water at one temperature into the inner glass and then run water at a different temperature over the surface of the outer glass. Which water should be hot and which should be cold?
A)fill the inner glass with cold water while running hot water over the outer glass
B)fill the inner glass with hot water while running cold water over the outer glass
C)submerge both glass in a tub of hot water; wait for a while and then transfer to a tub of cold water
D)submerge both glass in a tub of cold water; wait for a while and then transfer to a tub of hot water
A)fill the inner glass with cold water while running hot water over the outer glass
B)fill the inner glass with hot water while running cold water over the outer glass
C)submerge both glass in a tub of hot water; wait for a while and then transfer to a tub of cold water
D)submerge both glass in a tub of cold water; wait for a while and then transfer to a tub of hot water

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which temperature is the hottest?
A)100°C
B)100 K
C)100°F
D)They are all equal.
E)none of the above
A)100°C
B)100 K
C)100°F
D)They are all equal.
E)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following temperatures is not possible?
A)25 K
B)-200 K
C)-200°C
D)0°C
E)0°F
A)25 K
B)-200 K
C)-200°C
D)0°C
E)0°F

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
The value 300 joules could be a measure of ________.
A)the energy content of an object
B)the amount of heat transferred from one object to another
C)the temperature of an object
D)A and B
E)all of the above
A)the energy content of an object
B)the amount of heat transferred from one object to another
C)the temperature of an object
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
What is temperature a measure of?
A)Temperature is a measure of the average kinetic energy of the submicroscopic particles of a material.
B)Temperature is a measure of the total amount of energy found within a material.
C)Temperature is a measure of how hot or cold something is relative to some standard.
D)Both A and C are correct.
A)Temperature is a measure of the average kinetic energy of the submicroscopic particles of a material.
B)Temperature is a measure of the total amount of energy found within a material.
C)Temperature is a measure of how hot or cold something is relative to some standard.
D)Both A and C are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
A supersonic airplane heats up considerably when traveling through the air at speeds greater than the speed of sound. As a result, the airplane during supersonic flight is longer than when it is at rest on the ground. Offer an explanation for this length change from a submicroscopic perspective.
A)The engines on the supersonic plane are so powerful that they cause the plane's metal skin to stretch during accelerations.
B)Materials expand as they are heated.
C)Atmospheric air molecules hit the supersonic plane at great velocities causing the atoms of the planes metal skin to start moving faster.
D)Both A and B are correct.
A)The engines on the supersonic plane are so powerful that they cause the plane's metal skin to stretch during accelerations.
B)Materials expand as they are heated.
C)Atmospheric air molecules hit the supersonic plane at great velocities causing the atoms of the planes metal skin to start moving faster.
D)Both A and B are correct.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
Will your body possess energy after you die? If so, what kind?
A)Your body possesses no energy after you die because it cannot move on its own.
B)Your body possesses both potential and kinetic energy even as it lies still.
C)Your body possesses chemical potential energy, which is the potential energy found within the atoms and molecules of your tissues.
D)Your body possesses chemical potential energy, but also kinetic energy at the level of atoms and molecules, which vibrate rapidly.
A)Your body possesses no energy after you die because it cannot move on its own.
B)Your body possesses both potential and kinetic energy even as it lies still.
C)Your body possesses chemical potential energy, which is the potential energy found within the atoms and molecules of your tissues.
D)Your body possesses chemical potential energy, but also kinetic energy at the level of atoms and molecules, which vibrate rapidly.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
What determines the direction of heat flow?
A)Heat always flows from a lower-temperature substance into a higher-temperature substance.
B)Heat always flows from a higher-temperature substance into a lower-temperature substance.
C)Heat flow direction depends upon the density of the substances involved.
D)The direction of heat flow is not predictable in any given process.
A)Heat always flows from a lower-temperature substance into a higher-temperature substance.
B)Heat always flows from a higher-temperature substance into a lower-temperature substance.
C)Heat flow direction depends upon the density of the substances involved.
D)The direction of heat flow is not predictable in any given process.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
How many joules are there in a candy bar containing 230,000 calories?
A)230,000 joules
B)55,000 joules
C)460,000 joules
D)960,000 joules
A)230,000 joules
B)55,000 joules
C)460,000 joules
D)960,000 joules

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
When a liquid evaporates, the vapor expands because ________.
A)the gas particles have less kinetic energy than the liquid particles
B)the gas particles have less potential energy than the liquid particles
C)the gas particles have more potential energy than the liquid particles
D)the gas particles have more kinetic energy than the liquid particles
E)the gas particles are bigger than the liquid particles
A)the gas particles have less kinetic energy than the liquid particles
B)the gas particles have less potential energy than the liquid particles
C)the gas particles have more potential energy than the liquid particles
D)the gas particles have more kinetic energy than the liquid particles
E)the gas particles are bigger than the liquid particles

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
Heat Is a measure of ________.
A)temperature
B)internal thermal energy
C)average kinetic energy
D)none of the above
A)temperature
B)internal thermal energy
C)average kinetic energy
D)none of the above

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
A minimum temperature exists (absolute zero). Why does no known maximum temperature exist?
A)Scientists have not yet determined what instruments might be able to measure the maximum temperature.
B)Scientists disagree on the pressure conditions with which to measure the maximum temperature.
C)Assuming no limit on the relative speed of atoms and molecules, it follows that there is no maximum temperature.
D)The maximum temperature is known to exist on the sun but we have not been able to measure it yet.
A)Scientists have not yet determined what instruments might be able to measure the maximum temperature.
B)Scientists disagree on the pressure conditions with which to measure the maximum temperature.
C)Assuming no limit on the relative speed of atoms and molecules, it follows that there is no maximum temperature.
D)The maximum temperature is known to exist on the sun but we have not been able to measure it yet.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following would best describe heat?
A)Heat is energy that moves from high to low temperature objects.
B)Heat is a measure of the temperature of an object.
C)Heat can be measured with a thermometer.
D)Objects at the same temperature have the same amount of heat.
E)Heat is a measure of the average amount of energy in an object.
A)Heat is energy that moves from high to low temperature objects.
B)Heat is a measure of the temperature of an object.
C)Heat can be measured with a thermometer.
D)Objects at the same temperature have the same amount of heat.
E)Heat is a measure of the average amount of energy in an object.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
Which has more total energy: a cup of boiling water at 100°C or a swimming pool of slightly cooler water at 90°C?
A)The cup of boiling water at 100°C has more energy.
B)The swimming pool at 90°C has more energy.
C)They both have the same amount of energy because they are both made of water.
D)It is not possible to answer this question without knowing the ambient temperature surrounding each body of water.
A)The cup of boiling water at 100°C has more energy.
B)The swimming pool at 90°C has more energy.
C)They both have the same amount of energy because they are both made of water.
D)It is not possible to answer this question without knowing the ambient temperature surrounding each body of water.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
How many calories are there in a candy bar containing 230 Calories?
A)230 calories
B)2300 calories
C)23,000 calories
D)230,000 calories
A)230 calories
B)2300 calories
C)23,000 calories
D)230,000 calories

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


