Exam 2: Particles of Matter
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
A post-1982 penny is made with zinc, but its density is actually greater than that of zinc because ________.
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
D
Humidity is a measure of the amount of water vapor in the atmosphere. Why is humidity always very low inside your kitchen freezer?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
Imagine that you can see individual molecules. You watch a small collection of molecules that are moving around slowly while vibrating and bumping against each other. The slower moving molecules then start to line up, but as they do so their vibrations increase. Soon all the molecules are aligned and vibrating about fixed positions. What is happening?
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
A
If you drop a hot rock into a pail of water, the temperature of the rock and the water change until the two are equal. The rock cools and the water warms. Does this hold true if the hot rock is dropped into the Atlantic Ocean?
(Multiple Choice)
4.8/5  (31)
(31)
Red colored Kool-Aid crystals are added to a still glass of hot water. The same amount of crystals are added to a second still glass filled with the same amount of cold water. With no stirring, which of the following would occur?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is something that is best described as having potential energy?
(Multiple Choice)
4.9/5  (39)
(39)
A piece of aluminum weighing 10.0 grams is placed into a graduated cylinder that has an initial volume (before immersion)of 35.0 mL. After immersion, the volume on the cylinder reads 38.7 mL. What is the density of the metal sample?
(Multiple Choice)
4.9/5  (45)
(45)
You take 50 mL of small BB's and combine them with 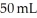 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
(Multiple Choice)
4.7/5  (38)
(38)
A supersonic airplane heats up considerably when traveling through the air at speeds greater than the speed of sound. As a result, the airplane during supersonic flight is longer than when it is at rest on the ground. Offer an explanation for this length change from a submicroscopic perspective.
(Multiple Choice)
4.7/5  (33)
(33)
A block of wood that weighs 10. g is immersed in water. The total amount of water displaced is 12 mL. What is the density of the block of wood?
(Multiple Choice)
4.8/5  (37)
(37)
What type of phase change does the following figure best describe? 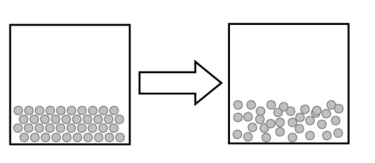
(Multiple Choice)
4.9/5  (41)
(41)
Someone wants to sell you a piece of gold that they say is nearly pure. Before buying the piece, you measure its mass to be 52.3 grams and you find that it displaces 4.16 mL of water. Calculate its density and assess its purity given the fact that pure gold has a density of 19.3 g/mL.
(Multiple Choice)
4.9/5  (44)
(44)
Assuming temperature remains constant, what happens to the density of a gas as the gas is compressed into a smaller volume?
(Multiple Choice)
4.8/5  (27)
(27)
If all of the above images represents the same material, which has the highest temperature?
(Multiple Choice)
4.9/5  (49)
(49)
A TV screen looked at from a distance appears as a smooth continuous flow of images. Up close, however, we see this is an illusion. What really exists are a series of tiny dots (pixels). This is similar to a chemist's view of matter in that ________.
(Multiple Choice)
4.7/5  (43)
(43)
Why is it not possible for the scanning probe microscope (SPM)to make images of the inside of an atom?
(Multiple Choice)
4.9/5  (39)
(39)
How does Aristotle's model of matter explain why the air over a flame is always moist?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 1 - 20 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)