Deck 13: Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/160
Play
Full screen (f)
Deck 13: Properties of Solutions
1
When solutions of strong electrolytes in water are formed,the ions are surrounded by water molecules. These interactions are described as a case of ________.
A)hydration
B)supersaturation
C)crystallization
D)dehydration
E)saturation
A)hydration
B)supersaturation
C)crystallization
D)dehydration
E)saturation
hydration
2
Hydration is a specific example of the phenomenon known generally as ________.
A)salutation
B)disordering
C)solvation
D)condensation
E)dilution
A)salutation
B)disordering
C)solvation
D)condensation
E)dilution
solvation
3
A solution with a concentration higher than the solubility allows is ________.
A)not possible
B)unsaturated
C)supercritical
D)saturated
E)supersaturated
A)not possible
B)unsaturated
C)supercritical
D)saturated
E)supersaturated
supersaturated
4
An unsaturated solution is one that ________.
A)has no double bonds
B)contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
C)has a concentration lower than the solubility limit
D)contains more dissolved solute than the solubility allows
E)contains no solute
A)has no double bonds
B)contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
C)has a concentration lower than the solubility limit
D)contains more dissolved solute than the solubility allows
E)contains no solute

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following is most soluble in water?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following is least soluble in water?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following substances is least likely to dissolve in water?
A)HOCH2CH2OH
B)CHCl3
C)O CH3(CH2)9CH
CH3(CH2)9CH
D)CH3(CH2)8CH2OH
E)CCl4
A)HOCH2CH2OH
B)CHCl3
C)O
 CH3(CH2)9CH
CH3(CH2)9CHD)CH3(CH2)8CH2OH
E)CCl4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following substances is more likely to dissolve in water?
A)HOCH2CH2OH
B)CHCl3
C)O CH3(CH2)9CH
CH3(CH2)9CH
D)CH3(CH2)8CH3
E)CCl4
A)HOCH2CH2OH
B)CHCl3
C)O
 CH3(CH2)9CH
CH3(CH2)9CHD)CH3(CH2)8CH3
E)CCl4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following substances is more likely to dissolve in CH3OH?
A)CCl4
B)Kr
C)N2
D)CH3CH2OH
E)H2
A)CCl4
B)Kr
C)N2
D)CH3CH2OH
E)H2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
10
The principal reason for the extremely low solubility of NaCl in benzene (C6H6)is the ________.
A)strong solvent-solvent interactions
B)hydrogen bonding in C6H6
C)strength of the covalent bond in NaCl
D)weak solvation of Na+ and Cl- by C6H6
E)increased disorder due to mixing of solute and solvent
A)strong solvent-solvent interactions
B)hydrogen bonding in C6H6
C)strength of the covalent bond in NaCl
D)weak solvation of Na+ and Cl- by C6H6
E)increased disorder due to mixing of solute and solvent

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
11
The dissolution of water in octane (C8H18)is principally prevented by ________.
A)London dispersion forces between octane molecules
B)hydrogen bonding between water molecules
C)dipole-dipole attraction between octane molecules
D)ion-dipole attraction between water and octane molecules
E)repulsion between like-charged water and octane molecules
A)London dispersion forces between octane molecules
B)hydrogen bonding between water molecules
C)dipole-dipole attraction between octane molecules
D)ion-dipole attraction between water and octane molecules
E)repulsion between like-charged water and octane molecules

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
12
Compounds composed of a salt and water combined in definite proportions are known as ________.
A)clathrates
B)homogenates
C)ionic solids
D)molecular solids
E)hydrates
A)clathrates
B)homogenates
C)ionic solids
D)molecular solids
E)hydrates

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
13
When argon is placed in a container of neon,the argon spontaneously disperses throughout the neon because ________.
A)of the large attractive forces between argon and neon atoms
B)of hydrogen bonding
C)a decrease in energy occurs when the two mix
D)the dispersion of argon atoms produces an increase in disorder
E)of solvent-solute interactions
A)of the large attractive forces between argon and neon atoms
B)of hydrogen bonding
C)a decrease in energy occurs when the two mix
D)the dispersion of argon atoms produces an increase in disorder
E)of solvent-solute interactions

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following substances is more likely to dissolve in CCl4?
A)CBr4
B)HBr
C)HCl
D)CH3CH2OH
E)NaCl
A)CBr4
B)HBr
C)HCl
D)CH3CH2OH
E)NaCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following substances is more likely to dissolve in benzene (C6H6)?
A)CH3CH2OH
B)NH3
C)NaCl
D)CCl4
E)HBr
A)CH3CH2OH
B)NH3
C)NaCl
D)CCl4
E)HBr

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following vitamins is water soluble?
A)A
B)B
C)K
D)D
E)E
A)A
B)B
C)K
D)D
E)E

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
17
A supersaturated solution ________.
A)is one with more than one solute
B)is one that has been heated
C)is one with a higher concentration than the solubility limit
D)must be in contact with undissolved solid
E)exists only in theory and cannot actually be prepared
A)is one with more than one solute
B)is one that has been heated
C)is one with a higher concentration than the solubility limit
D)must be in contact with undissolved solid
E)exists only in theory and cannot actually be prepared

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
18
The phrase "like dissolves like" refers to the fact that ________.
A)gases can only dissolve other gases
B)polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
C)solvents can only dissolve solutes of similar molar mass
D)condensed phases can only dissolve other condensed phases
E)polar solvents dissolve nonpolar solutes and vice versa
A)gases can only dissolve other gases
B)polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
C)solvents can only dissolve solutes of similar molar mass
D)condensed phases can only dissolve other condensed phases
E)polar solvents dissolve nonpolar solutes and vice versa

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is most soluble in hexane (C6H14)?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
20
In a saturated solution of a salt in water,________.
A)the rate of crystallization > the rate of dissolution
B)the rate of dissolution > the rate of crystallization
C)seed crystal addition may cause massive crystallization
D)the rate of crystallization = the rate of dissolution
E)addition of more water causes massive crystallization
A)the rate of crystallization > the rate of dissolution
B)the rate of dissolution > the rate of crystallization
C)seed crystal addition may cause massive crystallization
D)the rate of crystallization = the rate of dissolution
E)addition of more water causes massive crystallization

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
21
The magnitudes of Kf and of Kb depend on the identity of the ________.
A)solute
B)solvent
C)solution
D)solvent and on temperature
E)solute and solvent
A)solute
B)solvent
C)solution
D)solvent and on temperature
E)solute and solvent

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements is false?
A)Nonpolar liquids tend to be insoluble in polar liquids.
B)The weaker the attraction between the solute and solvent molecules, the greater the solubility.
C)Substances with similar intermolecular attractive forces tend to be soluble in one another.
D)The solubility of a gas increases in direct proportion to its partial pressure above the solution.
E)The solubility of gases in water decreases with increasing temperature.
A)Nonpolar liquids tend to be insoluble in polar liquids.
B)The weaker the attraction between the solute and solvent molecules, the greater the solubility.
C)Substances with similar intermolecular attractive forces tend to be soluble in one another.
D)The solubility of a gas increases in direct proportion to its partial pressure above the solution.
E)The solubility of gases in water decreases with increasing temperature.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
23
A 0.100 m solution of which one of the following solutes will have the highest vapor pressure?
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
24
What is the concentration in ppm of a solution which is prepared by dissolving 15 mg of NaCl in 200 mL water?
A)1.5 × 10-5
B)75
C)0.075
D)15
E)7.5 × 10-5
A)1.5 × 10-5
B)75
C)0.075
D)15
E)7.5 × 10-5

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
25
Molality is defined as the ________.
A)moles solute/moles solvent
B)moles solute/liters solution
C)moles solute/kg solution
D)moles solute/kg solvent
E)none (dimensionless)
A)moles solute/moles solvent
B)moles solute/liters solution
C)moles solute/kg solution
D)moles solute/kg solvent
E)none (dimensionless)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
26
A 0.200 m solution of which one of the following solutes will have the lowest vapor pressure?
A)LiCl
B)AlCl3
C)CaCl2
D)glucose
E)KCl
A)LiCl
B)AlCl3
C)CaCl2
D)glucose
E)KCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
27
As the concentration of a solute in a solution increases,the freezing point of the solution ________ and the vapor pressure of the solution ________.
A)increases, increases
B)increases, decreases
C)decreases, increases
D)decreases, decreases
E)decreases, is unaffected
A)increases, increases
B)increases, decreases
C)decreases, increases
D)decreases, decreases
E)decreases, is unaffected

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following concentration units varies with temperature?
A)molarity
B)mass percent
C)mole fraction
D)molality
E)all of the above
A)molarity
B)mass percent
C)mole fraction
D)molality
E)all of the above

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
29
A solution contains 11% by mass of sodium chloride.This means that ________.
A)there are 11 g of sodium chloride in in 1.0 mL of this solution
B)100 g of the solution contains 11 g of sodium chloride
C)100 mL of the solution contains 11 g of sodium chloride
D)the density of the solution is 11 g/mL
E)the molality of the solution is 11
A)there are 11 g of sodium chloride in in 1.0 mL of this solution
B)100 g of the solution contains 11 g of sodium chloride
C)100 mL of the solution contains 11 g of sodium chloride
D)the density of the solution is 11 g/mL
E)the molality of the solution is 11

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
30
If the partial pressure of oxygen in the air a diver breathes is too great,________.
A)respiratory tissue is damaged by oxidation
B)hyperventilation results
C)the urge to breathe is increased and excessive CO2 is removed from the body
D)the urge to breathe is reduced and not enough CO2 is removed from the body
E)No problems result from this situation.
A)respiratory tissue is damaged by oxidation
B)hyperventilation results
C)the urge to breathe is increased and excessive CO2 is removed from the body
D)the urge to breathe is reduced and not enough CO2 is removed from the body
E)No problems result from this situation.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
31
Pressure has an appreciable effect on the solubility of ________ in liquids.
A)gases
B)solids
C)liquids
D)salts
E)solids and liquids
A)gases
B)solids
C)liquids
D)salts
E)solids and liquids

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
32
A solution contains 15 ppm of benzene.The density of the solution is 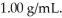 This means that ________.
This means that ________.
A)there are 15 mg of benzene in 1.0 g of this solution
B)100 g of the solution contains 15 g of benzene
C)1.0 g of the solution contains 15 × 10-6 g of benzene
D)1.0 L of the solution contains 15 g of benzene
E)the solution is 15% by mass of benzene
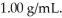 This means that ________.
This means that ________.A)there are 15 mg of benzene in 1.0 g of this solution
B)100 g of the solution contains 15 g of benzene
C)1.0 g of the solution contains 15 × 10-6 g of benzene
D)1.0 L of the solution contains 15 g of benzene
E)the solution is 15% by mass of benzene

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
33
The solubility of nitrogen gas at 25 °C and 1 atm is 6.8 × 10-4 mol/L.If the partial pressure of nitrogen gas in air is 0.76 atm,what is the concentration (molarity)of dissolved nitrogen?
A)6.8 × 10-4 M
B)5.2 × 10-4 M
C)4.9 × 10-4 M
D)3.8 × 10-4 M
E)1.1 × 10-5 M
A)6.8 × 10-4 M
B)5.2 × 10-4 M
C)4.9 × 10-4 M
D)3.8 × 10-4 M
E)1.1 × 10-5 M

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
34
The concentration of CO2 in a soft drink bottled with a partial pressure of CO2 of 6.5 atm over the liquid at 29 °C is 2.2 × 10-1 M.The Henry's law constant for CO2 at this temperature is ________.
A)2.2 × 10-1 mol/L-atm
B)7.6 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)3.4 × 10-2 mol/L-atm
E)More information is needed to solve the problem.
A)2.2 × 10-1 mol/L-atm
B)7.6 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)3.4 × 10-2 mol/L-atm
E)More information is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the molarity of a 10.0% (by mass)aqueous solution of hydrochloric acid.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble)
A)CCl4 < CHCl3 < NaNO3
B)CH3OH < CH4 < LiF
C)CH4 < NaNO3 < CHCl3
D)LiF < NaNO3 < CHCl3
E)CH3OH < Cl4 < CHCl3
A)CCl4 < CHCl3 < NaNO3
B)CH3OH < CH4 < LiF
C)CH4 < NaNO3 < CHCl3
D)LiF < NaNO3 < CHCl3
E)CH3OH < Cl4 < CHCl3

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
37
Which component of air is the primary problem in a condition known as "the bends"?
A)O2
B)CO2
C)He
D)N2
E)CO
A)O2
B)CO2
C)He
D)N2
E)CO

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the molarity of a 17.5% (by mass)aqueous solution of nitric acid.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
39
The concentration of CO2 in a soft drink bottled with a partial pressure of CO2 of 4.0 atm over the liquid at 25 °C is 1.2 × 10-1 M.The Henry's law constant for CO2 at this temperature is ________.
A)3.0 × 10-2 mol/L-atm
B)4.5 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)2.3 × 10-2 mol/L-atm
E)More information is needed to solve the problem.
A)3.0 × 10-2 mol/L-atm
B)4.5 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)2.3 × 10-2 mol/L-atm
E)More information is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
40
Of the concentration units below,only ________ uses kg of solvent in its calculation.
A)mass %
B)ppm
C)ppb
D)molarity
E)molality
A)mass %
B)ppm
C)ppb
D)molarity
E)molality

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
41
A 81.5 g sample of calcium chloride is dissolved in 102 g of water at 45 °C.The solution is cooled to 20.0 °C and no precipitate is observed.This solution is ________.
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol)in 920.0 ml of water at 25 °C.The vapor pressure of pure water at 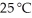 is
is 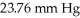 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.
A)0.278 mm Hg
B)0.605 mm Hg
C)22.98 mm Hg
D)23.48 mm Hg
E)23.76 mm Hg
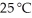 is
is 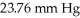 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.A)0.278 mm Hg
B)0.605 mm Hg
C)22.98 mm Hg
D)23.48 mm Hg
E)23.76 mm Hg

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following liquids will have the lowest freezing point?
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
44
The process of a substance sticking to the surface of another is called ________.
A)absorption
B)diffusion
C)effusion
D)adsorption
E)coagulation
A)absorption
B)diffusion
C)effusion
D)adsorption
E)coagulation

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
45
Of the following,a 0.1 M aqueous solution of ________ will have the highest freezing point.
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
46
All of the following are considered to be colloids except ________.
A)an emulsion
B)a homogeneous mixture
C)an aerosol
D)a foam
E)All of the above are colloids.
A)an emulsion
B)a homogeneous mixture
C)an aerosol
D)a foam
E)All of the above are colloids.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
47
The solubility of Ar in water at 25 °C is 1.6 × 10-3 M when the pressure of the Ar above the solution is 1.0 atm.The solubility of Ar at a pressure of 2.5 atm is ________ M.
A)1.6 × 103
B)6.4 × 10-4
C)4.0 × 10-3
D)7.5 × 10-2
E)1.6 × 10-3
A)1.6 × 103
B)6.4 × 10-4
C)4.0 × 10-3
D)7.5 × 10-2
E)1.6 × 10-3

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
48
A 2.05 m aqueous solution of some unknown had a boiling point of 102.1 °C.Which one of the following could be the unknown compound? The boiling point elevation constant for water is 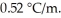
A)NaCl
B)CH3OH
C)C6H12O6
D)Na2CO3
E)CaBr2
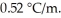
A)NaCl
B)CH3OH
C)C6H12O6
D)Na2CO3
E)CaBr2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
49
The process of solute particles being surrounded by solvent particles is known as ________.
A)salutation
B)agglomeration
C)solvation
D)agglutination
E)dehydration
A)salutation
B)agglomeration
C)solvation
D)agglutination
E)dehydration

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following liquids will have the highest freezing point?
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following aqueous solutions will have the highest boiling point?
A)0.10 m Na2SO4
B)0.20 m glucose
C)0.25 m sucrose
D)0.10 m NaCl
E)0.10 m SrSO4
A)0.10 m Na2SO4
B)0.20 m glucose
C)0.25 m sucrose
D)0.10 m NaCl
E)0.10 m SrSO4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
52
The most likely van't Hoff factor for an 0.01 m CaI2 solution is ________.
A)1.00
B)3.00
C)1.27
D)2.69
E)3.29
A)1.00
B)3.00
C)1.27
D)2.69
E)3.29

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
53
Pairs of liquids that will mix in all proportions are called ________ liquids.
A)miscible
B)unsaturated
C)polar liquids
D)saturated
E)supersaturated
A)miscible
B)unsaturated
C)polar liquids
D)saturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following liquids will have the highest freezing point?
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
55
The ratio of the actual value of a colligative property to the value calculated,assuming the substance to be a nonelectrolyte,is referred to as ________.
A)Henry's law
B)vapor pressure lowering
C)the van't Hoff factor
D)freezing point depression
E)osmotic pressure
A)Henry's law
B)vapor pressure lowering
C)the van't Hoff factor
D)freezing point depression
E)osmotic pressure

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
56
Colligative properties of solutions include all of the following except ________.
A)depression of vapor pressure upon addition of a solute to a solvent
B)elevation of the boiling point of a solution upon addition of a solute to a solvent
C)depression of the freezing point of a solution upon addition of a solute to a solvent
D)an increase in the osmotic pressure of a solution upon the addition of more solute
E)the increase of reaction rates with increase in temperature
A)depression of vapor pressure upon addition of a solute to a solvent
B)elevation of the boiling point of a solution upon addition of a solute to a solvent
C)depression of the freezing point of a solution upon addition of a solute to a solvent
D)an increase in the osmotic pressure of a solution upon the addition of more solute
E)the increase of reaction rates with increase in temperature

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrophobic colloids ________.
A)are those that contain water
B)can be stabilized by adsorption of ions
C)are those that do not contain water
D)can be stabilized by coagulation
E)will separate into two phases if they are stabilized
A)are those that contain water
B)can be stabilized by adsorption of ions
C)are those that do not contain water
D)can be stabilized by coagulation
E)will separate into two phases if they are stabilized

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
58
Of the following,a 0.1 M aqueous solution of ________ will have the lowest freezing point.
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following solutes has a limiting van't Hoff factor (i)of 3 when dissolved in water?
A)KNO3
B)CH3OH
C)CCl4
D)Na2SO4
E)sucrose
A)KNO3
B)CH3OH
C)CCl4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following liquids will have the lowest freezing point?
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
61
What is the molarity of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
A)143
B)2.45
C)2.56
D)2.23
E)1.43 × 10-2
A)143
B)2.45
C)2.56
D)2.23
E)1.43 × 10-2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
62
The concentration (M)of HCl in a solution prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ M.The density of the solution is 0.79 g/mL.
A)21
B)0.93
C)0.58
D)6.0 × 10-4
E)1.72
A)21
B)0.93
C)0.58
D)6.0 × 10-4
E)1.72

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
63
A 17.2 g sample of potassium chlorate is dissolved in 250 g of water at 65 °C.The solution is cooled to 30.0 °C and no precipitate is observed.This solution is ________.
A)hydrated
B)miscible
C)saturated
D)unsaturated
E)supersaturated
A)hydrated
B)miscible
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
64
The mole fraction of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 16 g of urea in 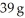 of H2O is ________.
of H2O is ________.
A)0.58
B)0.37
C)0.13
D)0.11
E)9.1
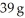 of H2O is ________.
of H2O is ________.A)0.58
B)0.37
C)0.13
D)0.11
E)9.1

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
65
What is the mole fraction of LiCl in solution that is 9.0% by mass LiCl and has a density of 1.00 g/mL?
A)0.960
B)2.33
C)0.0403
D)2.12
E)9.00
A)0.960
B)2.33
C)0.0403
D)2.12
E)9.00

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
66
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water.The density of the resulting solution is 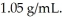 The concentration of CaCl2 in this solution is ________ molal.
The concentration of CaCl2 in this solution is ________ molal.
A)0.214
B)0.569
C)5.70
D)63.2
E)1.76
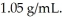 The concentration of CaCl2 in this solution is ________ molal.
The concentration of CaCl2 in this solution is ________ molal.A)0.214
B)0.569
C)5.70
D)63.2
E)1.76

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
67
The molarity of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 11 g of urea in 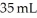 of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
A)0.314
B)5.24
C)314
D)0.00524
E)0.00642
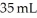 of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.A)0.314
B)5.24
C)314
D)0.00524
E)0.00642

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
68
A solution at 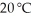 that is 3.75 M in MnSO4 monohydrate is considered a(n)________ solution.The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.
that is 3.75 M in MnSO4 monohydrate is considered a(n)________ solution.The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.
A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated
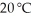 that is 3.75 M in MnSO4 monohydrate is considered a(n)________ solution.The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.
that is 3.75 M in MnSO4 monohydrate is considered a(n)________ solution.The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
69
A solution is prepared by dissolving 24.7 g of KBr in 375 g water at 20.0 °C.What is the molarity (M)of KBr in this solution? Density of the solution at 20.0 °C is 0.998 g/mL.
A)0.552
B)0.0659
C)0.000552
D)552
E)65.9
A)0.552
B)0.0659
C)0.000552
D)552
E)65.9

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
70
A 30.0 g sample of potassium nitrate is dissolved in 100 g of water at 60 °C.The solution is cooled to 20.0 °C and a small amount of precipitate is observed.This solution is ________.
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
71
The concentration of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 16 g of urea in 39 g of H2O is ________ molal.
A)96
B)6.8
C)0.68
D)6.3
E)0.11
A)96
B)6.8
C)0.68
D)6.3
E)0.11

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
72
What is the concentration (M)of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 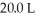 of water? (Assume volume does not change after adding HCl.)
of water? (Assume volume does not change after adding HCl.)
A)1.28
B)0.0350
C)35.0
D)3.50 × 10-5
E)14.0
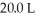 of water? (Assume volume does not change after adding HCl.)
of water? (Assume volume does not change after adding HCl.)A)1.28
B)0.0350
C)35.0
D)3.50 × 10-5
E)14.0

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
73
A solution is prepared by dissolving 15.8 g of MgCl2 in 255 g of water.What is the mole fraction of Cl- in this solution?
A)0.977
B)0.0116
C)0.0232
D)0.988
E)0.00583
A)0.977
B)0.0116
C)0.0232
D)0.988
E)0.00583

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
74
The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.A solution at 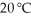 that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.
that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.
A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated
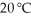 that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.
that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
75
The vapor pressure of pure ethanol at 60 °C is 0.459 atm.Raoult's Law predicts that a solution prepared by dissolving 10.0 mmol naphthalene (nonvolatile)in 90.0 mmol ethanol will have a vapor pressure of ________ atm.
A)0.498
B)0.413
C)0.790
D)0.367
E)0.0918
A)0.498
B)0.413
C)0.790
D)0.367
E)0.0918

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
76
What is the mole fraction of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 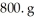 of water? The density of the solution is 1.0 g/mL.
of water? The density of the solution is 1.0 g/mL.
A)0.0155
B)0.0319
C)0.984
D)0.874
E)32.0
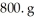 of water? The density of the solution is 1.0 g/mL.
of water? The density of the solution is 1.0 g/mL.A)0.0155
B)0.0319
C)0.984
D)0.874
E)32.0

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
77
A solution is prepared by dissolving 15.0 g of NH3 in 250.0 g of water.The density of the resulting solution is 0.974 g/mL.The molarity of NH3 in the solution is ________ M.
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
78
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water.The density of the resulting solution is 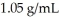 .The concentration of Cl- in this solution is ________ M.
.The concentration of Cl- in this solution is ________ M.
A)0.214
B)0.562
C)1.12
D)1.20
E)6.64 × 10-2
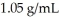 .The concentration of Cl- in this solution is ________ M.
.The concentration of Cl- in this solution is ________ M.A)0.214
B)0.562
C)1.12
D)1.20
E)6.64 × 10-2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
79
What is the molality of LiCl in solution that is 9.0% by mass LiCl and has a density of 1.00 g/mL?
A)9.00
B)2.12
C)2.33
D)90.0
E)0.0900
A)9.00
B)2.12
C)2.33
D)90.0
E)0.0900

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
80
A solution is prepared by dissolving 25.0 g of NaCl in 500.0 g of water.What is the molality (m)of NaCl in the solution? The density of the solution is 1.0 g/mL.
A)0.000856
B)0.0500
C)50.0
D)8.56
E)0.856
A)0.000856
B)0.0500
C)50.0
D)8.56
E)0.856

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck


