Exam 13: Properties of Solutions
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The concentration (M)of HCl in a solution prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ M.The density of the solution is 0.79 g/mL.
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
A solution is prepared by dissolving 15.8 g of MgCl2 in 255 g of water.What is the mole fraction of Cl- in this solution?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
The vapor pressure of pure water at 25 °C is 23.8 torr.What is the vapor pressure (torr)of water above a solution prepared by dissolving 18.0 g of glucose (a nonelectrolyte,MW = 180.0 g/mol)in 95.0 g of water?
Free
(Multiple Choice)
4.8/5  (27)
(27)
Correct Answer:
B
Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol)in 920.0 ml of water at 25 °C.The vapor pressure of pure water at 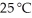 is
is 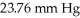 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.
(Multiple Choice)
4.9/5  (34)
(34)
A solution is prepared by dissolving 25.0 g of NaCl in 500.0 g of water.What is the molality (m)of NaCl in the solution? The density of the solution is 1.0 g/mL.
(Multiple Choice)
4.9/5  (39)
(39)
Which component of air is the primary problem in a condition known as "the bends"?
(Multiple Choice)
4.9/5  (31)
(31)
What is the mole fraction of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 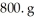 of water? The density of the solution is 1.0 g/mL.
of water? The density of the solution is 1.0 g/mL.
(Multiple Choice)
4.8/5  (38)
(38)
How many grams of solution are present if a solute is dissolved in 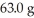 of water and the concentration is 12.7% by mass?
of water and the concentration is 12.7% by mass?
(Multiple Choice)
4.9/5  (38)
(38)
The process of solute particles being surrounded by solvent particles is known as ________.
(Multiple Choice)
4.8/5  (33)
(33)
The vapor pressure of pure ethanol at 60 °C is 0.459 atm.Raoult's Law predicts that a solution prepared by dissolving 10.0 mmol naphthalene (nonvolatile)in 90.0 mmol ethanol will have a vapor pressure of ________ atm.
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the freezing point of a 0.09500 m aqueous solution of glucose.The molal freezing-point-depression constant of water is 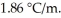
(Multiple Choice)
4.9/5  (32)
(32)
All of the following are considered to be colloids except ________.
(Multiple Choice)
4.9/5  (40)
(40)
What is the mole fraction of nitric acid of a 16.2% (by mass)aqueous solution of nitric acid?
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the mole fraction of phosphoric acid (H3PO4)in a 38.5% (by mass)aqueous solution.
(Multiple Choice)
4.7/5  (31)
(31)
Calculate the freezing point of a 0.05500 m aqueous solution of NaN 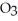 .The molal freezing-point-depression constant of water is
.The molal freezing-point-depression constant of water is 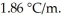 (Assume 100% ionization of NaNO3.)
(Assume 100% ionization of NaNO3.)
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following substances is more likely to dissolve in water?
(Multiple Choice)
4.7/5  (36)
(36)
The mole fraction of NaCl in water is 0.0195.Calculate the vapor pressure lowering (in mm Hg)of the solution at 25.0 °C.(Note: The vapor pressure of pure water at 25.0 °C is 23.76 mm Hg.)
(Short Answer)
5.0/5  (27)
(27)
Showing 1 - 20 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)