Deck 38: Photons: Light Waves Behaving As Particles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 38: Photons: Light Waves Behaving As Particles
1
A metal having a work function of 2.8 eV is illuminated with monochromatic light whose photon energy is 3.9 eV.What is the threshold frequency for photoelectron production? 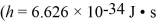 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
A) 6.8 × 1014 Hz
B) 2.7 × 1014 Hz
C) 7.6 × 1014 Hz
D) 8.5 × 1014 Hz
E) 9.4 × 1014 Hz
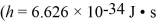 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)A) 6.8 × 1014 Hz
B) 2.7 × 1014 Hz
C) 7.6 × 1014 Hz
D) 8.5 × 1014 Hz
E) 9.4 × 1014 Hz
6.8 × 1014 Hz
2
Gamma rays are photons with very high energy.How many visible-light photons with a wavelength of 500 nm would you need to match the energy of a gamma-ray photon with energy 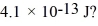 (h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
A) 1.0 × 106
B) 1.4 × 108
C) 6.2 × 109
D) 3.9 × 103
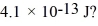 (h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)A) 1.0 × 106
B) 1.4 × 108
C) 6.2 × 109
D) 3.9 × 103
1.0 × 106
3
In a photoelectric effect experiment,electrons emerge from a copper surface with a maximum kinetic energy of 1.10 eV when light shines on the surface.The work function of copper is 4.65 eV.Which one of the following values is closest to the wavelength of the light? 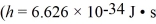 ,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
A) 220 nm
B) 150 nm
C) 360 nm
D) 1100 nm
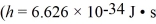 ,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)A) 220 nm
B) 150 nm
C) 360 nm
D) 1100 nm
220 nm
4
If the accuracy in measuring the position of a particle increases,the accuracy in measuring its velocity will
A) increase.
B) decrease.
C) remain the same.
D) It is impossible to say since the two measurements are independent and do not affect each other.
A) increase.
B) decrease.
C) remain the same.
D) It is impossible to say since the two measurements are independent and do not affect each other.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
A light beam from a 2.1-mW He-Ne laser has a wavelength of 633 nm.How many photons does the laser emit in one second? (h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s)
A) 6.7 × 1015
B) 8.8 × 1015
C) 1.1 × 1016
D) 1.3 × 1016
A) 6.7 × 1015
B) 8.8 × 1015
C) 1.1 × 1016
D) 1.3 × 1016

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
A metal having a work function of 2.4 eV is illuminated with monochromatic light whose photon energy is 4.0 eV.What is the maximum kinetic energy of the photoelectrons produced by this light? (h = 6.626 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
A) 2.6 × 10-19 J
B) 3.8 × 10-19 J
C) 4.7 × 10-19 J
D) 5.5 × 10-19 J
E) 6.4 × 10-19 J
A) 2.6 × 10-19 J
B) 3.8 × 10-19 J
C) 4.7 × 10-19 J
D) 5.5 × 10-19 J
E) 6.4 × 10-19 J

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
A metal having a work function of 2.5 eV is illuminated with white light that has a continuous wavelength band from 400 nm to 700 nm.For which one of the following ranges of the wavelength band in this white light are photoelectrons NOT produced? 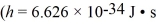 , c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
, c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
A) 500 nm to 700 nm
B) 400 nm to 560 nm
C) 500 nm to 560 nm
D) 400 nm to 500 nm
E) 560 nm to 700 nm
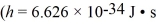 , c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
, c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)A) 500 nm to 700 nm
B) 400 nm to 560 nm
C) 500 nm to 560 nm
D) 400 nm to 500 nm
E) 560 nm to 700 nm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
Upon being struck by 240-nm photons,a metal ejects electrons with a maximum kinetic energy of 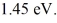 What is the work function of this metal? (h = 6.626 × 10-34 J • s,
What is the work function of this metal? (h = 6.626 × 10-34 J • s, 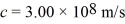 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
A) 3.73 eV
B) 3.13 eV
C) 4.33 eV
D) 4.92 eV
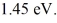 What is the work function of this metal? (h = 6.626 × 10-34 J • s,
What is the work function of this metal? (h = 6.626 × 10-34 J • s, 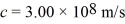 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)A) 3.73 eV
B) 3.13 eV
C) 4.33 eV
D) 4.92 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
A photon of initial wavelength 0.651 nm,after being scattered from a free electron at rest,moves off at an angle of 120° with respect to its incident direction. 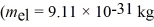 ,
, 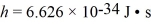 ,
, 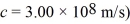 (a)What is the wavelength of the scattered photon?
(a)What is the wavelength of the scattered photon?
(b)What is the energy of the scattered photon?
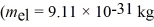 ,
, 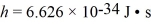 ,
, 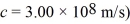 (a)What is the wavelength of the scattered photon?
(a)What is the wavelength of the scattered photon?(b)What is the energy of the scattered photon?

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
A stopping potential of 0.50 V is required when a phototube is illuminated with monochromatic light of wavelength 590 nm.Monochromatic light of a different wavelength is now shown on the tube,and the stopping potential is measured to be 2.30 V.What is the wavelength of this new light? (c = 3.00 × 108 m/s, 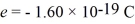 ,
, 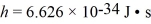 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
A) 320 nm
B) 300 nm
C) 340 nm
D) 360 nm
E) 410 nm
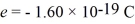 ,
, 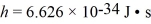 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)A) 320 nm
B) 300 nm
C) 340 nm
D) 360 nm
E) 410 nm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
When a certain metal is illuminated by light,photoelectrons are observed provided that the wavelength of the light is less than 669 nm.Which one of the following values is closest to the work function of this metal? (h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s, 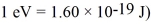
A) 1.9 eV
B) 2.0 eV
C) 2.2 eV
D) 2.3 eV
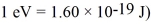
A) 1.9 eV
B) 2.0 eV
C) 2.2 eV
D) 2.3 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
In a particular case of Compton scattering,a photon collides with a free electron and scatters backwards.The wavelength after the collision is exactly double the wavelength before the collision.What is the wavelength of the incident photon? (mel = 9.11 × 10-31 kg, 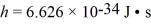 ,c = 3.00 × 108 m/s)
,c = 3.00 × 108 m/s)
A) 3.6 pm
B) 4.8 pm
C) 2.4 pm
D) 1.2 pm
E) 6.0 pm
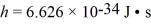 ,c = 3.00 × 108 m/s)
,c = 3.00 × 108 m/s)A) 3.6 pm
B) 4.8 pm
C) 2.4 pm
D) 1.2 pm
E) 6.0 pm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
A metal surface has a work function of 1.50 eV.Calculate the maximum kinetic energy,in eV,of electrons ejected from this surface by electromagnetic radiation of wavelength 311 nm. 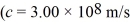 ,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
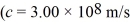 ,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
A beam of red light and a beam of violet light each deliver the same power on a surface.For which beam is the number of photons hitting the surface per second the greatest?
A) the red beam
B) the violet beam
C) It is the same for both beams.
A) the red beam
B) the violet beam
C) It is the same for both beams.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
Light of wavelength 400 nm falls on a metal surface having a work function 1.70 eV.What is the maximum kinetic energy of the photoelectrons emitted from the metal? 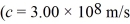 ,h = 6.626 × 10-34 J • s = 4.141 × 10-15 ev • s,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s = 4.141 × 10-15 ev • s,1 eV = 1.60 × 10-19 J)
A) 4.52 eV
B) 3.11 eV
C) 1.41 eV
D) 2.82 eV
E) 1.70 eV
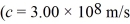 ,h = 6.626 × 10-34 J • s = 4.141 × 10-15 ev • s,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s = 4.141 × 10-15 ev • s,1 eV = 1.60 × 10-19 J)A) 4.52 eV
B) 3.11 eV
C) 1.41 eV
D) 2.82 eV
E) 1.70 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
When a metal surface is illuminated with light of wavelength 437 nm,the stopping potential for photoelectrons is 1.67 V.(c = 3.00 × 108 m/s, 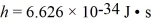 ,
, 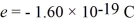 ,
,
1 eV = 1.60 × 10-19 J,mel = 9.11 × 10-31 kg)
(a)What is the work function of the metal,in eV?
(b)What is the maximum speed of the ejected electrons?
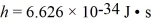 ,
, 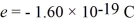 ,
,1 eV = 1.60 × 10-19 J,mel = 9.11 × 10-31 kg)
(a)What is the work function of the metal,in eV?
(b)What is the maximum speed of the ejected electrons?

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
If the accuracy in measuring the velocity of a particle increases,the accuracy in measuring its position will
A) increase.
B) decrease.
C) remain the same.
D) It is impossible to say since the two measurements are independent and do not affect each other.
A) increase.
B) decrease.
C) remain the same.
D) It is impossible to say since the two measurements are independent and do not affect each other.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
An 84-kW AM radio station broadcasts at 1000 kHz .How many photons are emitted each second by the transmitting antenna? (h = 6.626 × 10-34 J • s)
A) 1.3 ×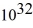
B) 2.9 ×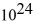
C) 6.3 ×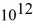
D) 1.4 ×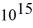
A) 1.3 ×
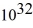
B) 2.9 ×
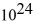
C) 6.3 ×
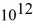
D) 1.4 ×

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
A laser emits light of wavelength 463 nm during a brief pulse that lasts for 25 ms and has a total energy of 1.2 J.How many photons are emitted in that single pulse? 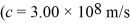 , h = 6.626 × 10-34 J • s)
, h = 6.626 × 10-34 J • s)
A) 2.8 × 1018
B) 6.9 × 1019
C) 3.4 × 1019
D) 1.1 × 1017
E) 2.2 × 1017
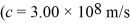 , h = 6.626 × 10-34 J • s)
, h = 6.626 × 10-34 J • s)A) 2.8 × 1018
B) 6.9 × 1019
C) 3.4 × 1019
D) 1.1 × 1017
E) 2.2 × 1017

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
Monochromatic light strikes a metal surface and electrons are ejected from the metal.If the intensity of the light is increased,what will happen to the ejection rate and maximum energy of the electrons?
A) greater ejection rate; same maximum energy
B) same ejection rate; greater maximum energy
C) greater ejection rate; greater maximum energy
D) same ejection rate; same maximum energy
A) greater ejection rate; same maximum energy
B) same ejection rate; greater maximum energy
C) greater ejection rate; greater maximum energy
D) same ejection rate; same maximum energy

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
An unstable particle produced in a high-energy collision is measured to have an energy of 483 MeV and an uncertainty in energy of 84 keV.Use the Heisenberg uncertainty principle to estimate the lifetime of this particle.( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
A nonrelativistic electron is confined to a length of 500 pm on the x-axis.What is the kinetic energy of the electron if its speed is equal to the minimum uncertainty possible in its speed? (h = 1.055 × 10-34 J • s,mel = 9.11 × 10-31 kg,1 eV = 1.60 × 10-19 J)
A) 0.00038 eV
B) 0.0038 eV
C) 0.038 eV
D) 0.38 eV
E) 3.8 eV
A) 0.00038 eV
B) 0.0038 eV
C) 0.038 eV
D) 0.38 eV
E) 3.8 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
A small dust particle of mass 7.90 × 10-6 g is being observed under a magnifying lens.Its position is determined to within 0.0050 mm.(1 y = 3.156 × 107 s, 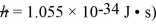 (a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(b)Assuming the dust particle is moving at the speed you just found,how many years would it take for the particle to move 1.0 mm?
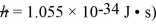 (a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.
(a)Find the minimum uncertainty in its velocity implied by the uncertainty in its position.(b)Assuming the dust particle is moving at the speed you just found,how many years would it take for the particle to move 1.0 mm?

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
A laser produces a beam of 4000-nm light.A shutter allows a pulse of light,30 ps in duration,to pass.Which of the following is closest to the uncertainty in the energy of a photon in the pulse? ( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)
A) 10-6 eV
B) 10-5 eV
C) 10-4 eV
D) 10-3 eV
E) 10-2 eV
A) 10-6 eV
B) 10-5 eV
C) 10-4 eV
D) 10-3 eV
E) 10-2 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? 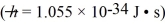
A) 0.12 m/s
B) 1.2 m/s
C) 12 m/s
D) 0.012 m/s
E) 0.0012 m/s
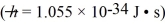
A) 0.12 m/s
B) 1.2 m/s
C) 12 m/s
D) 0.012 m/s
E) 0.0012 m/s

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
An ultraviolet source produces a monochromatic beam of 200-nm light.A shutter allows a pulse to pass that is 10,000 wavelengths long.The uncertainty in the energy of a photon in this pulse is closest to which of the following? ( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s, 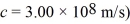
A) 5 × 10-7 eV
B) 5 × 10-6 eV
C) 5 × 10-5 eV
D) 5 × 10-4 eV
E) 5 × 10-3 eV
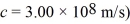
A) 5 × 10-7 eV
B) 5 × 10-6 eV
C) 5 × 10-5 eV
D) 5 × 10-4 eV
E) 5 × 10-3 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
The lifetime of an excited nuclear state is 1.0 ns.What is the minimum uncertainty in the energy of this state? ( h = 1.055 × 10-34 J • s = 6.591 × 10-16 eV • s, 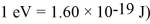
A) 5.0 × 10-10 eV
B) 5.0 × 10-26 eV
C) 3.3 × 10-25 eV
D) 1.6 × 10-7 eV
E) 3.3 × 10-7 eV
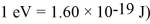
A) 5.0 × 10-10 eV
B) 5.0 × 10-26 eV
C) 3.3 × 10-25 eV
D) 1.6 × 10-7 eV
E) 3.3 × 10-7 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
A 440-nm spectral line is produced by a transition from an excited state to the ground state.The natural line width of the spectral line is 0.020 pm.The average time the atom spends in the excited state is closest to which of the following? ( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)
A) 2.5 × 10-6 s
B) 2.5 × 10-7 s
C) 2.5 × 10-8 s
D) 2.5 × 10-9 s
E) 2.5 × 10-10 s
A) 2.5 × 10-6 s
B) 2.5 × 10-7 s
C) 2.5 × 10-8 s
D) 2.5 × 10-9 s
E) 2.5 × 10-10 s

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
A beam of X-rays at a certain wavelength are scattered from a free electron at rest and the scattered beam is observed at 45.0° to the incident beam.What is the change in the wavelength of the X-rays? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s)
A) 0.175 pm
B) 0.276 pm
C) 0.000 pm
D) 0.356 pm
E) 0.710 pm
A) 0.175 pm
B) 0.276 pm
C) 0.000 pm
D) 0.356 pm
E) 0.710 pm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%.What is the minimum uncertainty in its position? ( h = 1.055 × 10-34 J • s,mel = 9.11 × 10-31 kg)
A) 0.15 nm
B) 0.29 nm
C) 0.44 nm
D) 0.60 nm
E) 0.80 nm
A) 0.15 nm
B) 0.29 nm
C) 0.44 nm
D) 0.60 nm
E) 0.80 nm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
A nonrelativistic proton is confined to a length of 2.0 pm on the x-axis.What is the kinetic energy of the proton if its speed is equal to the minimum uncertainty possible in its speed? 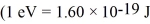 ,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)
A) 0.13 eV
B) 1.3 eV
C) 13 eV
D) 130 eV
E) 1300 eV
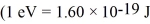 ,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)A) 0.13 eV
B) 1.3 eV
C) 13 eV
D) 130 eV
E) 1300 eV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
X-rays of energy 2.9 × 104 eV are scattered by a free stationary electron through an angle of 135°.What is the energy of the scattered X-rays,in electron volts? (mel = 9.11 × 10-31 kg, 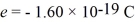 ,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
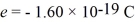 ,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s,1 eV = 1.60 × 10-19 J)
Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
A certain particle's energy is measured by a detector to within 1.0 × 10-18.What is the minimum uncertainty we can have in its arrival time at the detector? 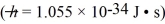
A) 5.3 × 10-16 s
B) 5.3 × 10-15 s
C) 5.3 × 10-14 s
D) 5.3 × 10-13 s
E) 5.3 × 10-17 s
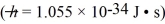
A) 5.3 × 10-16 s
B) 5.3 × 10-15 s
C) 5.3 × 10-14 s
D) 5.3 × 10-13 s
E) 5.3 × 10-17 s

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 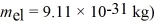
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
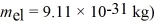
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
A photon of wavelength 18.0 pm is scattered through an angle of 120° by a stationary electron.What is the wavelength of the scattered photon? (mel = 9.11 × 10-31 kg, 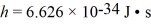 ,c = 3.00 × 108 m/s)
,c = 3.00 × 108 m/s)
A) 19.2 pm
B) 20.4 pm
C) 21.6 pm
D) 22.9 pm
E) 24.1 pm
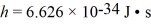 ,c = 3.00 × 108 m/s)
,c = 3.00 × 108 m/s)A) 19.2 pm
B) 20.4 pm
C) 21.6 pm
D) 22.9 pm
E) 24.1 pm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
The excited state of a certain atom is 3.2 eV ± 0.21 eV. 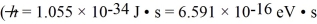 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
(a)What is the average lifetime of this state?
(b)If the excited energy were doubled to 6.4 eV ± 0.21 eV,how would the lifetime be affected?
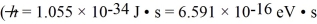 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)(a)What is the average lifetime of this state?
(b)If the excited energy were doubled to 6.4 eV ± 0.21 eV,how would the lifetime be affected?

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
The energy of an electron state has an uncertainty of 0.500 eV.What is the minimum uncertainty in the lifetime of the level? ( h = 1.055 × 10-34 J • s = 6.591 × 10-16 eV • s, 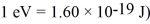
A) 6.59 × 10-16 s
B) 4.14 × 10-15 s
C) 6.59 × 10-12 s
D) 4.14 × 10-11 s
E) 6.59 × 10-9 s
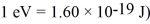
A) 6.59 × 10-16 s
B) 4.14 × 10-15 s
C) 6.59 × 10-12 s
D) 4.14 × 10-11 s
E) 6.59 × 10-9 s

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
A photon of wavelength 29 pm is scattered by a stationary electron.What is the maximum possible energy loss of the photon? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s, 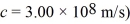
A) 4.0 keV
B) 7.0 keV
C) 10 keV
D) 6.1 keV
E) 12 keV
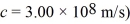
A) 4.0 keV
B) 7.0 keV
C) 10 keV
D) 6.1 keV
E) 12 keV

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck


