Exam 38: Photons: Light Waves Behaving As Particles
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
A beam of X-rays at a certain wavelength are scattered from a free electron at rest and the scattered beam is observed at 45.0° to the incident beam.What is the change in the wavelength of the X-rays? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s)
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
E
A metal having a work function of 2.8 eV is illuminated with monochromatic light whose photon energy is 3.9 eV.What is the threshold frequency for photoelectron production? 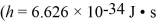 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
A photon of initial wavelength 0.651 nm,after being scattered from a free electron at rest,moves off at an angle of 120° with respect to its incident direction. 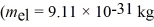 ,
, 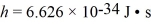 ,
, 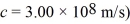 (a)What is the wavelength of the scattered photon?
(b)What is the energy of the scattered photon?
(a)What is the wavelength of the scattered photon?
(b)What is the energy of the scattered photon?
Free
(Short Answer)
4.9/5  (26)
(26)
Correct Answer:
(a)0.655 nm
(b)3.04 × 10-16 J
A laser produces a beam of 4000-nm light.A shutter allows a pulse of light,30 ps in duration,to pass.Which of the following is closest to the uncertainty in the energy of a photon in the pulse? ( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)
(Multiple Choice)
4.7/5  (34)
(34)
An unstable particle produced in a high-energy collision is measured to have an energy of 483 MeV and an uncertainty in energy of 84 keV.Use the Heisenberg uncertainty principle to estimate the lifetime of this particle.( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)
(Short Answer)
4.8/5  (33)
(33)
A light beam from a 2.1-mW He-Ne laser has a wavelength of 633 nm.How many photons does the laser emit in one second? (h = 6.626 × 10-34 J • s,c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (27)
(27)
An 84-kW AM radio station broadcasts at 1000 kHz .How many photons are emitted each second by the transmitting antenna? (h = 6.626 × 10-34 J • s)
(Multiple Choice)
4.9/5  (30)
(30)
A laser emits light of wavelength 463 nm during a brief pulse that lasts for 25 ms and has a total energy of 1.2 J.How many photons are emitted in that single pulse? 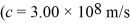 , h = 6.626 × 10-34 J • s)
, h = 6.626 × 10-34 J • s)
(Multiple Choice)
4.8/5  (35)
(35)
A beam of red light and a beam of violet light each deliver the same power on a surface.For which beam is the number of photons hitting the surface per second the greatest?
(Multiple Choice)
4.7/5  (39)
(39)
A measurement of an electron's speed is 2.0 × 106 m/s and has an uncertainty of 10%.What is the minimum uncertainty in its position? ( h = 1.055 × 10-34 J • s,mel = 9.11 × 10-31 kg)
(Multiple Choice)
4.8/5  (34)
(34)
Monochromatic light strikes a metal surface and electrons are ejected from the metal.If the intensity of the light is increased,what will happen to the ejection rate and maximum energy of the electrons?
(Multiple Choice)
4.9/5  (28)
(28)
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? 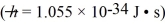
(Multiple Choice)
4.8/5  (29)
(29)
In a particular case of Compton scattering,a photon collides with a free electron and scatters backwards.The wavelength after the collision is exactly double the wavelength before the collision.What is the wavelength of the incident photon? (mel = 9.11 × 10-31 kg, 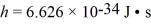 ,c = 3.00 × 108 m/s)
,c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (42)
(42)
A metal surface has a work function of 1.50 eV.Calculate the maximum kinetic energy,in eV,of electrons ejected from this surface by electromagnetic radiation of wavelength 311 nm. 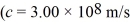 ,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,e = - 1.60 × 10-19 C,1 eV = 1.60 × 10-19 J)
(Short Answer)
4.8/5  (41)
(41)
A certain particle's energy is measured by a detector to within 1.0 × 10-18.What is the minimum uncertainty we can have in its arrival time at the detector? 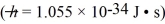
(Multiple Choice)
4.9/5  (35)
(35)
The excited state of a certain atom is 3.2 eV ± 0.21 eV. 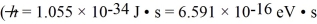 ,1 eV = 1.60 × 10-19 J)
(a)What is the average lifetime of this state?
(b)If the excited energy were doubled to 6.4 eV ± 0.21 eV,how would the lifetime be affected?
,1 eV = 1.60 × 10-19 J)
(a)What is the average lifetime of this state?
(b)If the excited energy were doubled to 6.4 eV ± 0.21 eV,how would the lifetime be affected?
(Essay)
4.9/5  (37)
(37)
A photon of wavelength 29 pm is scattered by a stationary electron.What is the maximum possible energy loss of the photon? (mel = 9.11 × 10-31 kg,h = 6.626 × 10-34 J • s, 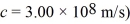
(Multiple Choice)
4.8/5  (41)
(41)
The energy of an electron state has an uncertainty of 0.500 eV.What is the minimum uncertainty in the lifetime of the level? ( h = 1.055 × 10-34 J • s = 6.591 × 10-16 eV • s, 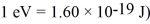
(Multiple Choice)
4.8/5  (38)
(38)
If the accuracy in measuring the position of a particle increases,the accuracy in measuring its velocity will
(Multiple Choice)
5.0/5  (36)
(36)
Upon being struck by 240-nm photons,a metal ejects electrons with a maximum kinetic energy of 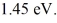 What is the work function of this metal? (h = 6.626 × 10-34 J • s,
What is the work function of this metal? (h = 6.626 × 10-34 J • s, 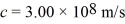 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 38
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)