Deck 19: Entropy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 19: Entropy
1
When an ideal gas increases in volume at constant pressure,the average kinetic energy of the gas molecules
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.
increases.
2
Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 2.0 L,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa
33 kPa
3
A container is filled with a mixture of helium (light molecules)and oxygen (heavy molecules)gases.A thermometer in the container reads 22°C.Which gas molecules have the greater average kinetic energy?
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
It is the same for both of the gases because the temperatures are the same.
4
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket.The open end of the tube is under the water of a lake,as shown in the figure.When the lower end of the tube is just under the surface of the lake,where the temperature is 37°C and the pressure is 1.0 × 105 Pa,the air pocket occupies a volume of 630 cm3. Suppose now that the lower end of the tube is at a depth of 86 m in the lake,where the temperature is 7.0°C.What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 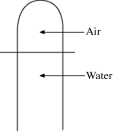


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
If the temperature of a fixed amount of an ideal gas is increased,it NECESSARILY follows that
A) the pressure of the gas will increase.
B) the volume of the gas will increase.
C) the speed of the gas molecules will increase.
D) All of the above statements are correct.
A) the pressure of the gas will increase.
B) the volume of the gas will increase.
C) the speed of the gas molecules will increase.
D) All of the above statements are correct.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
The average molecular kinetic energy of a gas can be determined by knowing
A) only the number of molecules in the gas.
B) only the volume of the gas.
C) only the pressure of the gas.
D) only the temperature of the gas.
E) All of the above quantities must be known to determine the average molecular kinetic energy.
A) only the number of molecules in the gas.
B) only the volume of the gas.
C) only the pressure of the gas.
D) only the temperature of the gas.
E) All of the above quantities must be known to determine the average molecular kinetic energy.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
A bag of potato chips contains 2.00 L of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0°C. What will be the volume of the air in the bag if you take it with you,still sealed,to the mountains where the temperature is 7.00°C and atmospheric pressure is 70.0 kPa? Assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air.
(1 atm = 1.01 × 105 Pa)
A) 4.13 L
B) 1.01 L
C) 1.38 L
D) 2.76 L
(1 atm = 1.01 × 105 Pa)
A) 4.13 L
B) 1.01 L
C) 1.38 L
D) 2.76 L

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
If we double the root-mean-square speed (thermal speed)of the molecules of a gas,then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of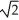
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of
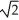
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
An ideal gas is kept in a rigid container that expands negligibly when heated.The gas starts at a temperature of 20.0°C,and heat is added to increase its temperature.At what temperature will its root-mean-square speed (thermal speed)be double its value at 20.0°C?
A) 40.0°C
B) 141°C
C) 313°C
D) 400°C
E) 899°C
A) 40.0°C
B) 141°C
C) 313°C
D) 400°C
E) 899°C

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
The figure shows a 50-kg frictionless cylindrical piston that floats on 0.68 mol of compressed air at 30°C. How far does the piston move if the temperature is increased to 300°C? 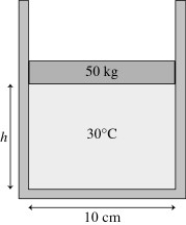
A) 120 cm
B) 250 cm
C) 130 cm
D) 1300 cm

A) 120 cm
B) 250 cm
C) 130 cm
D) 1300 cm

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
A weather balloon contains 12.0 m3 of hydrogen gas when the balloon is released from a location at which the temperature is 22.0°C and the pressure is 101 kPa.The balloon rises to a location where the temperature is -30.0°C and the pressure is 20.0 kPa.If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure,what is the new volume of the balloon? Assume that in both cases the hydrogen gas is in thermal equilibrium with the outside air.
A) 14.0 m3
B) 2.38 m3
C) 49.9 m3
D) 82.6 m3
E) 4.16 m3
A) 14.0 m3
B) 2.38 m3
C) 49.9 m3
D) 82.6 m3
E) 4.16 m3

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
The root-mean-square speed (thermal speed)for a certain gas at 100°C is 0.500 km/s.If the temperature of the gas is now increased to 200°C,the root-mean-square (thermal)speed will be closest to
A) 563 m/s.
B) 634 m/s.
C) 707 m/s.
D) 804 m/s.
E) 1000 m/s.
A) 563 m/s.
B) 634 m/s.
C) 707 m/s.
D) 804 m/s.
E) 1000 m/s.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
What is the average translational kinetic energy per molecule of an ideal gas at a temperature of
300 K? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J
300 K? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
For a fixed amount of gas,if the absolute temperature of the gas is doubled,what happens to the pressure of the gas?
A) The answer cannot be determined without volume information.
B) The pressure of the gas becomes double the original pressure.
C) The pressure of the gas becomes eight times the original pressure.
D) The pressure of the gas becomes one half the original pressure.
E) The pressure of the gas becomes four times the original pressure.
A) The answer cannot be determined without volume information.
B) The pressure of the gas becomes double the original pressure.
C) The pressure of the gas becomes eight times the original pressure.
D) The pressure of the gas becomes one half the original pressure.
E) The pressure of the gas becomes four times the original pressure.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature.What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It becomes 4 times as great.
C) It becomes 2 times as great.
D) It becomes 1/2 as great.
E) It becomes 1/4 as great.
A) It does not change.
B) It becomes 4 times as great.
C) It becomes 2 times as great.
D) It becomes 1/2 as great.
E) It becomes 1/4 as great.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in pressure.If the original root-mean-square speed (thermal speed)of the gas molecules was V,the new speed is
A) V.
B) 2V.
C)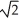 V.
V.
D) V/2.
E) V/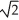
A) V.
B) 2V.
C)
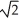 V.
V.D) V/2.
E) V/
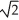

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
A sealed 26-m3 tank is filled with 2000 moles of oxygen gas (O2)at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.The final pressure of the gas is closest to
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
A container is filled with a mixture of helium (light molecules)and oxygen (heavy molecules)gases.A thermometer in the container reads 22°C.Which gas molecules have the greater average speed?
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
A) It is the same for both of the gases because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
An ideal gas is at a pressure 1.00 × 105 N/m2 and occupies a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
A) 0.500 × 105 N/m2
B) 4.00 × 105 N/m2
C) 1.00 × 105 N/m2
D) 2.00 × 105 N/m2
E) The answer depends on the mass of the gas particles.
A) 0.500 × 105 N/m2
B) 4.00 × 105 N/m2
C) 1.00 × 105 N/m2
D) 2.00 × 105 N/m2
E) The answer depends on the mass of the gas particles.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
A fixed amount of ideal gas is held in a rigid container that expands negligibly when heated.At 20°C the gas pressure is p.If we add enough heat to increase the temperature from 20°C to 40°C,the pressure will be
A) impossible to determine since we do not know the number of moles of gas in the container.
B) greater than 2p.
C) less than 2p.
D) equal to 2p.
E) impossible to determine since we do not know the volume of gas in the container.
A) impossible to determine since we do not know the number of moles of gas in the container.
B) greater than 2p.
C) less than 2p.
D) equal to 2p.
E) impossible to determine since we do not know the volume of gas in the container.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
At what temperature would the root-mean-square speed (thermal speed)of oxygen molecules be 13.0 m/s? Assume that oxygen approximates an ideal gas.The mass of one O2 molecule is 5.312 × 10-26 kg.The Boltzmann constant is 1.38 × 10-23 J/K.
A) 0.217 K
B) 1800 K
C) 5410 K
D) 0.0666 K
A) 0.217 K
B) 1800 K
C) 5410 K
D) 0.0666 K

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
The root-mean-square speed (thermal speed)of the molecules of a gas is 200 m/s at a temperature 23.0°C. What is the mass of the individual molecules? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 2.13 × 10-25 kg
B) 2.45 × 10-25 kg
C) 5.66 × 10-25 kg
D) 1.78 × 10-25 kg
E) 3.11 × 10-25 kg
A) 2.13 × 10-25 kg
B) 2.45 × 10-25 kg
C) 5.66 × 10-25 kg
D) 1.78 × 10-25 kg
E) 3.11 × 10-25 kg

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
What is the average kinetic energy of an ideal gas molecule at 569°C? (The Boltzmann constant is
1)38 × 10-23 J/K.)
A) 1.74 × 10-20 J
B) 5.81 × 10-21 J
C) 1.18 × 10-17 J
D) 3.93 × 10-19 J
1)38 × 10-23 J/K.)
A) 1.74 × 10-20 J
B) 5.81 × 10-21 J
C) 1.18 × 10-17 J
D) 3.93 × 10-19 J

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
At 50.0°C,the average translational kinetic energy of a gas molecule is K.If the temperature is now increased to 100.0°C,the average translational kinetic energy of a molecule will be closest to
A) 1.07K.
B) 1.15K.
C) 1.41K.
D) 2.00K.
E) 4.00K.
A) 1.07K.
B) 1.15K.
C) 1.41K.
D) 2.00K.
E) 4.00K.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
Dust particles are pulverized rock,which has density 2500 kg/m3.They are approximately spheres
20 μm in diameter.Treating dust as an ideal gas,what is the root-mean-square speed (thermal speed)of a dust particle at 400°C? (The Boltzmann constant is 1.38 × 10-23 J/K.)
A) 5.2 × 10-5 m/s
B) 1.7 × 10-5 m/s
C) 3.0 × 10-5 m/s
D) 7.3 × 10-5 m/s
20 μm in diameter.Treating dust as an ideal gas,what is the root-mean-square speed (thermal speed)of a dust particle at 400°C? (The Boltzmann constant is 1.38 × 10-23 J/K.)
A) 5.2 × 10-5 m/s
B) 1.7 × 10-5 m/s
C) 3.0 × 10-5 m/s
D) 7.3 × 10-5 m/s

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed)of 200 m/s.The mass of a helium molecule is 3.40 × 10-27 kg.What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is
1)38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.
A) 3.39 kPa
B) 1.13 kPa
C) 570 Pa
D) 2.26 kPa
E) 9.10 Pa
1)38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.
A) 3.39 kPa
B) 1.13 kPa
C) 570 Pa
D) 2.26 kPa
E) 9.10 Pa

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
The root-mean-square speed (thermal speed)of the molecules of a gas is 200 m/s at 23.0°C. At 227°C the root-mean-square speed (thermal speed)of the molecules will be closest to
A) 160 m/s
B) 330 m/s
C) 260 m/s
D) 630 m/s
E) 2000 m/s
A) 160 m/s
B) 330 m/s
C) 260 m/s
D) 630 m/s
E) 2000 m/s

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


