Exam 19: Entropy
Exam 1: Foundations48 Questions
Exam 1: A: Foundations2 k Questions
Exam 2: Motion in One Dimension15 Questions
Exam 3: Acceleration48 Questions
Exam 4: Momentum7 Questions
Exam 5: Energy15 Questions
Exam 6: Principle of Relativity5 Questions
Exam 7: Interactions7 Questions
Exam 8: Force76 Questions
Exam 9: Work48 Questions
Exam 10: Motion in a Plane134 Questions
Exam 11: Motion in a Circle54 Questions
Exam 12: Torque68 Questions
Exam 13: Gravity44 Questions
Exam 14: Special Relativity49 Questions
Exam 15: Periodic Motion49 Questions
Exam 16: Waves in One Dimension23 Questions
Exam 17: Waves in Two and Three Dimensions44 Questions
Exam 18: Fluids54 Questions
Exam 19: Entropy27 Questions
Exam 20: Energy Transferred Thermally30 Questions
Exam 21: Degradation of Energy39 Questions
Exam 22: Electric Interactions20 Questions
Exam 23: The Electric Field34 Questions
Exam 24: Gausss Law46 Questions
Exam 25: Work and Energy in Electrostatics53 Questions
Exam 26: Charge Separation and Storage33 Questions
Exam 27: Magnetic Interactions37 Questions
Exam 28: Magnetic Fields of Charged Particles in Motion35 Questions
Exam 29: Changing Magnetic Fields38 Questions
Exam 30: Changing Electric Fields30 Questions
Exam 31: Electric Circuits68 Questions
Exam 32: Electronics46 Questions
Exam 33: Ray Optics65 Questions
Exam 34: Wave and Particle Optics100 Questions
Select questions type
A weather balloon contains 12.0 m3 of hydrogen gas when the balloon is released from a location at which the temperature is 22.0°C and the pressure is 101 kPa.The balloon rises to a location where the temperature is -30.0°C and the pressure is 20.0 kPa.If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure,what is the new volume of the balloon? Assume that in both cases the hydrogen gas is in thermal equilibrium with the outside air.
Free
(Multiple Choice)
4.8/5  (23)
(23)
Correct Answer:
C
Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 2.0 L,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
A
A container is filled with a mixture of helium (light molecules)and oxygen (heavy molecules)gases.A thermometer in the container reads 22°C.Which gas molecules have the greater average kinetic energy?
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
A
A fixed amount of ideal gas is held in a rigid container that expands negligibly when heated.At 20°C the gas pressure is p.If we add enough heat to increase the temperature from 20°C to 40°C,the pressure will be
(Multiple Choice)
4.8/5  (38)
(38)
The root-mean-square speed (thermal speed)for a certain gas at 100°C is 0.500 km/s.If the temperature of the gas is now increased to 200°C,the root-mean-square (thermal)speed will be closest to
(Multiple Choice)
4.8/5  (41)
(41)
An ideal gas is at a pressure 1.00 × 105 N/m2 and occupies a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
(Multiple Choice)
4.7/5  (37)
(37)
If the temperature of a fixed amount of an ideal gas is increased,it NECESSARILY follows that
(Multiple Choice)
4.9/5  (29)
(29)
The figure shows a 50-kg frictionless cylindrical piston that floats on 0.68 mol of compressed air at 30°C. How far does the piston move if the temperature is increased to 300°C? 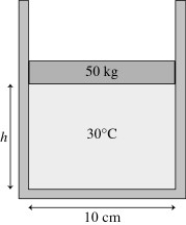
(Multiple Choice)
5.0/5  (34)
(34)
An ideal gas is kept in a rigid container that expands negligibly when heated.The gas starts at a temperature of 20.0°C,and heat is added to increase its temperature.At what temperature will its root-mean-square speed (thermal speed)be double its value at 20.0°C?
(Multiple Choice)
4.9/5  (54)
(54)
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed)of 200 m/s.The mass of a helium molecule is 3.40 × 10-27 kg.What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is
1)38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.
(Multiple Choice)
4.9/5  (43)
(43)
A container is filled with a mixture of helium (light molecules)and oxygen (heavy molecules)gases.A thermometer in the container reads 22°C.Which gas molecules have the greater average speed?
(Multiple Choice)
4.8/5  (35)
(35)
At what temperature would the root-mean-square speed (thermal speed)of oxygen molecules be 13.0 m/s? Assume that oxygen approximates an ideal gas.The mass of one O2 molecule is 5.312 × 10-26 kg.The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (29)
(29)
A sealed 26-m3 tank is filled with 2000 moles of oxygen gas (O2)at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.The final pressure of the gas is closest to
(Multiple Choice)
4.8/5  (39)
(39)
The average molecular kinetic energy of a gas can be determined by knowing
(Multiple Choice)
4.7/5  (33)
(33)
For a fixed amount of gas,if the absolute temperature of the gas is doubled,what happens to the pressure of the gas?
(Multiple Choice)
4.8/5  (38)
(38)
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket.The open end of the tube is under the water of a lake,as shown in the figure.When the lower end of the tube is just under the surface of the lake,where the temperature is 37°C and the pressure is 1.0 × 105 Pa,the air pocket occupies a volume of 630 cm3. Suppose now that the lower end of the tube is at a depth of 86 m in the lake,where the temperature is 7.0°C.What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 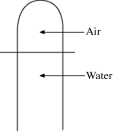
(Short Answer)
4.8/5  (37)
(37)
The root-mean-square speed (thermal speed)of the molecules of a gas is 200 m/s at 23.0°C. At 227°C the root-mean-square speed (thermal speed)of the molecules will be closest to
(Multiple Choice)
4.9/5  (34)
(34)
If we double the root-mean-square speed (thermal speed)of the molecules of a gas,then
(Multiple Choice)
4.8/5  (36)
(36)
What is the average translational kinetic energy per molecule of an ideal gas at a temperature of
300 K? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (38)
(38)
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature.What happens to the average speed of the molecules in the sample?
(Multiple Choice)
4.9/5  (26)
(26)
Showing 1 - 20 of 27
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)