Deck 5: Stoichiometry: Quantitative Information About Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 5: Stoichiometry: Quantitative Information About Chemical Reactions
1
What mass of iron can be produced from the reaction of 175 kg Fe2O3 with 385 kg CO?
Fe2O3(s)+ 3 CO(g) 2 Fe(s)+ 3 CO2(g)
A) 2.19 kg
B) 30.6 kg
C) 61.2 kg
D) 122 kg
E) 512 kg
Fe2O3(s)+ 3 CO(g) 2 Fe(s)+ 3 CO2(g)
A) 2.19 kg
B) 30.6 kg
C) 61.2 kg
D) 122 kg
E) 512 kg
122 kg
2
Nitric oxide is made from the oxidation of ammonia.What mass of nitric oxide can be made from the reaction of 8.00 g NH3 with 17.0 g O2?
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g
12.8 g
3
When strongly heated,boric acid breaks down to boric oxide and water.What mass of boric oxide is formed from the decomposition of 15.0 g B(OH)3?
2 B(OH)3(s) B2O3(s)+ 3 H2O(g)
A) 7.50 g
B) 15.0 g
C) 8.44 g
D) 16.9 g
E) 33.8 g
2 B(OH)3(s) B2O3(s)+ 3 H2O(g)
A) 7.50 g
B) 15.0 g
C) 8.44 g
D) 16.9 g
E) 33.8 g
8.44 g
4
Magnesium reacts with iodine gas at high temperatures to form magnesium iodide.What mass of MgI2 can be produced from the reaction of 5.15 g Mg and 50.0 g I2?
A) 29.5 g
B) 44.9 g
C) 54.8 g
D) 55.2 g
E) 58.9 g
A) 29.5 g
B) 44.9 g
C) 54.8 g
D) 55.2 g
E) 58.9 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
The complete combustion of 0.32 moles of propane,C3H8,will
A) consume 0.32 mol O2 and produce 0.32 mol CO2.
B) consume 0.32 mol O2 and produce 0.64 mol CO2.
C) consume 1.6 mol O2 and produce 0.96 mol CO2.
D) consume 1.6 mol O2 and produce 4.8 mol CO2.
E) consume 1.6 mol O2 and produce 1.3 mol CO2.
A) consume 0.32 mol O2 and produce 0.32 mol CO2.
B) consume 0.32 mol O2 and produce 0.64 mol CO2.
C) consume 1.6 mol O2 and produce 0.96 mol CO2.
D) consume 1.6 mol O2 and produce 4.8 mol CO2.
E) consume 1.6 mol O2 and produce 1.3 mol CO2.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
Hydrogen peroxide decomposes into oxygen and water.What mass of oxygen is formed from the decomposition of 125 g of H2O2?
A) 58.8 g
B) 66.4 g
C) 107 g
D) 118 g
E) 125 g
A) 58.8 g
B) 66.4 g
C) 107 g
D) 118 g
E) 125 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
Sulfur dioxide will react with water to form sulfurous acid (see balanced equation below).
SO2(g)+ H2O(l) H2SO3(l)
What mass of sulfur dioxide is needed to prepare 21.06 g of H2SO3(l)?
A) 16.44 g
B) 26.98 g
C) 0.3287 g
D) 0.2566 g
E) 21.06 g
SO2(g)+ H2O(l) H2SO3(l)
What mass of sulfur dioxide is needed to prepare 21.06 g of H2SO3(l)?
A) 16.44 g
B) 26.98 g
C) 0.3287 g
D) 0.2566 g
E) 21.06 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
How many moles of sodium bromide can be produced from the reaction of 1.03 moles of sodium with 0.650 moles of bromine gas?
2 Na(s)+ Br2(g) 2 NaBr(s)
A) 0.650 mol
B) 1.03 mol
C) 1.30 mol
D) 2.06 mol
E) 0.515 mol
2 Na(s)+ Br2(g) 2 NaBr(s)
A) 0.650 mol
B) 1.03 mol
C) 1.30 mol
D) 2.06 mol
E) 0.515 mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
If 48.8 g of O2 is mixed with 48.8 g of H2 and the mixture is ignited,what is the maximum mass of water that may be produced?
A) 439 g
B) 54.9 g
C) 48.8 g
D) 98 g
E) 86.8 g
A) 439 g
B) 54.9 g
C) 48.8 g
D) 98 g
E) 86.8 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
The commercial production of phosphoric acid,H3PO4,can be represented by the equation
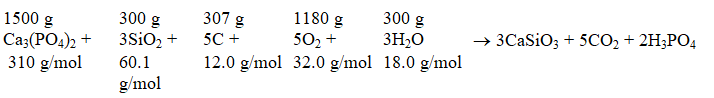
The molar mass for each reactant is shown the reactant,and the mass of each reactant for this problem is given Which substance is the limiting reactant?
A) H2O
B) C
C) O2
D) Ca3(PO4)2
E) SiO2
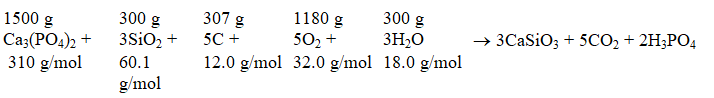
The molar mass for each reactant is shown the reactant,and the mass of each reactant for this problem is given Which substance is the limiting reactant?
A) H2O
B) C
C) O2
D) Ca3(PO4)2
E) SiO2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
What mass of copper(I)sulfide is required in order to prepare 0.610 kg of copper metal?
A) 0.610 kg
B) 0.305 kg
C) 0.459 kg
D) 1.53 kg
E) 0.764 kg
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
What mass of copper(I)sulfide is required in order to prepare 0.610 kg of copper metal?
A) 0.610 kg
B) 0.305 kg
C) 0.459 kg
D) 1.53 kg
E) 0.764 kg

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
How many grams of dioxygen are required to completely burn 4.3 g of C2H5OH?
A) 19 g
B) 15 g
C) 3.0 g
D) 42 g
E) 58 g
A) 19 g
B) 15 g
C) 3.0 g
D) 42 g
E) 58 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced?
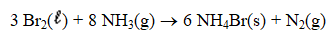
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
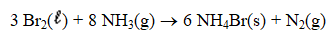
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
How many moles of Mg3P2(s)can be produced from the reaction of 0.14 mol Mg(s)with 0.020 mol P4(s)?
6 Mg(s)+ P4(s) 2 Mg3P2(s)
A) 0.047 mol
B) 0.14 mol
C) 0.42 mol
D) 0.020 mol
E) 0.040 mol
6 Mg(s)+ P4(s) 2 Mg3P2(s)
A) 0.047 mol
B) 0.14 mol
C) 0.42 mol
D) 0.020 mol
E) 0.040 mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
The compound P4S3 is used in matches.It reacts with oxygen to produce P4O10 and SO2.The chemical equation is shown below.
P4S3(s)+ O2(g) P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
A) 0.134 g
B) 1.20 g
C) 0.0389 g
D) 0.117 g
E) 0.350 g
P4S3(s)+ O2(g) P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
A) 0.134 g
B) 1.20 g
C) 0.0389 g
D) 0.117 g
E) 0.350 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the number of moles of O2 required to react with phosphorus to produce 4.76 g of P4O6.
(Molar mass P4O6 = 219.9 g/mol)
A) 0.0216 mol
B) 0.149 mol
C) 0.0649 mol
D) 0.0433 mol
E) 0.130 mol
(Molar mass P4O6 = 219.9 g/mol)
A) 0.0216 mol
B) 0.149 mol
C) 0.0649 mol
D) 0.0433 mol
E) 0.130 mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
If the complete combustion of an unknown mass of ethylene produces 16.0 g CO2,what mass of ethylene is combusted?
C2H4(g)+ 3 O2(g) 2 CO2(g)+ 2 H2O(g)
A) 0.182 g
B) 0.364 g
C) 5.10 g
D) 8.00 g
E) 12.6 g
C2H4(g)+ 3 O2(g) 2 CO2(g)+ 2 H2O(g)
A) 0.182 g
B) 0.364 g
C) 5.10 g
D) 8.00 g
E) 12.6 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
Potassium hydrogen carbonate decomposes with heat as follows:
2KHCO3(s) K2CO3(s)+ CO2(g)+ H2O(l)
How many moles of potassium carbonate will be produced if 201 g of potassium hydrogen carbonate are heated?
A) 1.00 mol
B) 1.45 mol
C) 10.8 mol
D) 101 mol
E) 2.01 mol
2KHCO3(s) K2CO3(s)+ CO2(g)+ H2O(l)
How many moles of potassium carbonate will be produced if 201 g of potassium hydrogen carbonate are heated?
A) 1.00 mol
B) 1.45 mol
C) 10.8 mol
D) 101 mol
E) 2.01 mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
Aspirin (C9H8O4)is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3( ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3(
 ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation:
Rh2(SO4)3(aq)+ 6NaOH(aq) → 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 6.20 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
A) 3.86 g
B) 12.4 g
C) 6.20 g
D) 0.966 g
E) 7.73 g
Rh2(SO4)3(aq)+ 6NaOH(aq) → 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 6.20 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
A) 3.86 g
B) 12.4 g
C) 6.20 g
D) 0.966 g
E) 7.73 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3(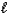 ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 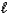
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3(
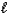 ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 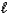
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
Complete combustion of a 0.30-mol sample of a hydrocarbon,CxHy,gives 1.80 mol of CO2 and 1.20 mol of H2O.The molecular formula of the original hydrocarbon is
A) C3H8.
B) C6H8.
C) C7H18.
D) C5H5.
E) C5H6.
A) C3H8.
B) C6H8.
C) C7H18.
D) C5H5.
E) C5H6.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
A 1.850 g mixture of SrCO3 and SrO is heated.The SrCO3 decomposes to SrO and CO2.What was the mass percentage of SrCO3 in the mixture if the mass after heating is 1.445 g?
A) 26.6%
B) 21.9%
C) 13.7%
D) 73.4%
E) 78.1%
A) 26.6%
B) 21.9%
C) 13.7%
D) 73.4%
E) 78.1%

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
Of the following,the only empirical formula is
A) C6H12.
B) C2H8.
C) C3H8.
D) H2O2.
E) O3.
A) C6H12.
B) C2H8.
C) C3H8.
D) H2O2.
E) O3.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
Sulfur hexafluoride is produced by reacting elemental sulfur with fluorine gas.S8(s)+ 24 F2(g) 8 SF6(g)
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
A) 40.2%
B) 45.8%
C) 47.6%
D) 54.6%
E) 61.0%
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
A) 40.2%
B) 45.8%
C) 47.6%
D) 54.6%
E) 61.0%

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
Chlorine was passed over 2.02 g of heated titanium,and 6.50 g of a chloride-containing compound of Ti was obtained.What is the empirical formula of the chloride-containing compound?
A) TiCl2
B) TiCl4
C) TiCl
D) TiCl3
E) Ti2Cl3
A) TiCl2
B) TiCl4
C) TiCl
D) TiCl3
E) Ti2Cl3

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
A compound consists of only C and F.It contains 38.71% C by mass.What is the empirical formula of the compound?
A) CF
B) CF2
C) CF3
D) C2F
E) CF4
A) CF
B) CF2
C) CF3
D) C2F
E) CF4

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
Under certain conditions the reaction of ammonia with excess oxygen will produce a 29.5% yield of NO.What mass of NH3 must react with excess oxygen to yield 157 g NO?
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
A) 89.1 g
B) 302 g
C) 263 g
D) 26.3 g
E) 938 g
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
A) 89.1 g
B) 302 g
C) 263 g
D) 26.3 g
E) 938 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
A 3.361 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 6.295 g CO2 and 3.866 g H2O.What is the empirical formula of the hydrocarbon?
A) CH3
B) CH2
C) C2H3
D) CH4
E) CH
A) CH3
B) CH2
C) C2H3
D) CH4
E) CH

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
A 5.95-g sample of AgNO3 is reacted with excess BaCl2 according to the equation
2AgNO3(aq)+ BaCl2(aq) 2AgCl(s)+ Ba(NO3)2(aq)
To give 3.75 g of AgCl.What is the percent yield of AgCl?
A) 49.8 %
B) 37.4 %
C) 74.7 %
D) 63.0 %
E) 100 %
2AgNO3(aq)+ BaCl2(aq) 2AgCl(s)+ Ba(NO3)2(aq)
To give 3.75 g of AgCl.What is the percent yield of AgCl?
A) 49.8 %
B) 37.4 %
C) 74.7 %
D) 63.0 %
E) 100 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
A certain compound has a molar mass of 150 g/mol.Which is a possible empirical formula for this compound?
A) CH2O
B) CHO
C) C2H2O2
D) C2HO
E) C2H2O
A) CH2O
B) CHO
C) C2H2O2
D) C2HO
E) C2H2O

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
The empirical formula of ethylene is CH2.An experimental determination of the molar mass of ethylene yields the value of 28 g/mol.What is the formula of ethylene?
A) C5H10
B) CH2
C) C2H4
D) C3H8
E) C6H9
A) C5H10
B) CH2
C) C2H4
D) C3H8
E) C6H9

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
The reaction of 11.9 g of CHCl3 with excess chlorine produced 10.3 g of CCl4,carbon tetrachloride:
2CHCl3 + 2Cl2 2CCl4 + 2HCl
What is the percent yield?
A) 86.6 %
B) 100 %
C) 67.2 %
D) 33.6 %
E) 44.8 %
2CHCl3 + 2Cl2 2CCl4 + 2HCl
What is the percent yield?
A) 86.6 %
B) 100 %
C) 67.2 %
D) 33.6 %
E) 44.8 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
A 2.841 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 7.794 g CO2 and water.What mass of hydrogen is contained in the original sample?
A) 0.7140 g
B) 4.953 g
C) 10.64 g
D) 2.826 g
E) 1.421 g
A) 0.7140 g
B) 4.953 g
C) 10.64 g
D) 2.826 g
E) 1.421 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the fermentation reaction of glucose:
C6H12O6 2C2H5OH + 2CO2
A 1.00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 67.8 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
A) 73.6 %
B) 36.8 %
C) 67.8 %
D) 100 %
E) none of these
C6H12O6 2C2H5OH + 2CO2
A 1.00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 67.8 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
A) 73.6 %
B) 36.8 %
C) 67.8 %
D) 100 %
E) none of these

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
Nitric oxide,NO,is made from the oxidation of NH3 as follows:
4NH3 + 5O2 4NO + 6H2O
If 9.4-g of NH3 gives 12.0 g of NO,what is the percent yield of NO?
A) 91 %
B) 44 %
C) 17 %
D) 72 %
E) 22 %
4NH3 + 5O2 4NO + 6H2O
If 9.4-g of NH3 gives 12.0 g of NO,what is the percent yield of NO?
A) 91 %
B) 44 %
C) 17 %
D) 72 %
E) 22 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
If 15.0 g N2 and 2.00 g H2 react to produce 1.38 g NH3,what is the percent yield of the reaction?
N2(g)+ 3 H2(g) 2 NH3(g)
A) 7.57%
B) 12.2%
C) 8.12%
D) 15.1%
E) 8.17%
N2(g)+ 3 H2(g) 2 NH3(g)
A) 7.57%
B) 12.2%
C) 8.12%
D) 15.1%
E) 8.17%

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
Sulfur trioxide,SO3,is made from the oxidation of SO2as follows: 2SO2 + O2 2SO3
A 21-g sample of SO2 gives 18 g of SO3.The of SO3 is .
A) 11 %
B) 69 %
C) 17 %
D) 26 %
E) 100 %
A 21-g sample of SO2 gives 18 g of SO3.The of SO3 is .
A) 11 %
B) 69 %
C) 17 %
D) 26 %
E) 100 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows:
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
If the reaction of 0.630 kg of copper(I)sulfide with excess oxygen produces 0.190 kg of copper metal,what is the percent yield?
A) 75.5 %
B) 39.9 %
C) 30.2 %
D) 151 %
E) 37.8 %
Cu2S(s)+ O2(g) 2Cu(s)+ SO2(g)
If the reaction of 0.630 kg of copper(I)sulfide with excess oxygen produces 0.190 kg of copper metal,what is the percent yield?
A) 75.5 %
B) 39.9 %
C) 30.2 %
D) 151 %
E) 37.8 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction of 0.779 g K with O2 forms 1.417 g potassium superoxide,a substance used in self-contained breathing devices.Determine the formula for potassium superoxide.
A) KO2
B) KO
C) K2O3
D) K2O
E) KO4
A) KO2
B) KO
C) K2O3
D) K2O
E) KO4

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
What volume of 2.52 M HCl is required to prepare 176.5 mL of 0.449 M HCl?
A) 9.91 102 mL
B) 1.56 102 mL
C) 31.4 mL
D) 0.0318 mL
E) 2.00 102 mL
A) 9.91 102 mL
B) 1.56 102 mL
C) 31.4 mL
D) 0.0318 mL
E) 2.00 102 mL

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
Naphthalene,a hydrocarbon,has an approximate molar mass of 128 g/mole.If the combustion of 0.6400 g naphthalene produces 0.3599 g H2O and 2.1977 g CO2,what is the molecular formula of this compound?
A) C8H32
B) C9H18
C) C9H20
D) C10H8
E) C11H7
A) C8H32
B) C9H18
C) C9H20
D) C10H8
E) C11H7

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
A mass of 0.4113 g of an unknown acid,HA,is titrated with NaOH.If the acid reacts with 28.10 mL of 0.1055 M NaOH(aq),what is the molar mass of the acid?
A) 2.965 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol
A) 2.965 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
The pH of a vinegar solution is 4.15.What is the H3O+ concentration of the solution?
A) 7.1 10-5 M
B) 1.6 10-2 M
C) 0.62 M
D) 1.4 M
E) 1.4 104 M
A) 7.1 10-5 M
B) 1.6 10-2 M
C) 0.62 M
D) 1.4 M
E) 1.4 104 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
How many moles of sulfate ions are there in a 0.387-L solution of 0.927 M Al2(SO4)3?
A) 0.359 mol
B) 1.08 mol
C) 1.25 mol
D) 0.120 mol
E) 7.19 mol
A) 0.359 mol
B) 1.08 mol
C) 1.25 mol
D) 0.120 mol
E) 7.19 mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
An aqueous nitric acid solution has a pH of 2.15.What mass of HNO3 is present in 20.0 L of this solution?
A) 0.11 g
B) 0.022 g
C) 3.7 g
D) 6.8 g
E) 8.9 g
A) 0.11 g
B) 0.022 g
C) 3.7 g
D) 6.8 g
E) 8.9 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
What minimum mass of copper (II)nitrate must be added to 30.0 mL of a 0.0387 M phosphate solution in order to completely precipitate all of the phosphate as solid copper (II)phosphate?
2PO43-(aq)+ 3Cu(NO3)2(aq) Cu3(PO4)2(s)+ 6NO3-(aq)
A) 0.218 g
B) 0.653 g
C) 0.145 g
D) 0.0726 g
E) 0.327 g
2PO43-(aq)+ 3Cu(NO3)2(aq) Cu3(PO4)2(s)+ 6NO3-(aq)
A) 0.218 g
B) 0.653 g
C) 0.145 g
D) 0.0726 g
E) 0.327 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
A dilute solution is prepared by transferring 45.00 mL of a 0.5616 M stock solution to a 400.0 mL volumetric flask and diluting to mark.What is the molarity of this dilute solution?
A) 0.06318 M
B) 0.1264 M
C) 0.04992 M
D) 0.01580 M
E) 0.2808 M
A) 0.06318 M
B) 0.1264 M
C) 0.04992 M
D) 0.01580 M
E) 0.2808 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
Polyethylene is a polymer consisting of only carbon and hydrogen.If 2.300 g of the polymer is burned in oxygen it produces 2.955 g H2O and 7.217 g CO2.What is the empirical formula of polyethylene?
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
Zn reacts with hydrochloric acid.
Zn(s)+ 2 HCl(aq) ZnCl2(aq)+ H2(g)
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
A) 0.251 L
B) 4.01 L
C) 0.125 L
D) 0.0627 L
E) 2.33 L
Zn(s)+ 2 HCl(aq) ZnCl2(aq)+ H2(g)
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
A) 0.251 L
B) 4.01 L
C) 0.125 L
D) 0.0627 L
E) 2.33 L

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
A 4.541 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 8.674 g CO2 and 5.328 g H2O.What is the empirical formula of the compound?
A) CHO
B) C2H6O
C) C3H6O
D) C3H4O
E) C3H8O3
A) CHO
B) C2H6O
C) C3H6O
D) C3H4O
E) C3H8O3

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pH of 5.3 10-3 M HCl(aq)?
A) -1.01
B) 2.28
C) 3.50
D) 1.01
E) 5.65
A) -1.01
B) 2.28
C) 3.50
D) 1.01
E) 5.65

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
The pH of an aqueous NaOH solution is 12.83.What is the hydrogen ion concentration of this solution?
A) 1.5 10-13 M
B) 6.7 10-2 M
C) 1.2 M
D) 6.7 1012 M
E) 2.7 10-6 M
A) 1.5 10-13 M
B) 6.7 10-2 M
C) 1.2 M
D) 6.7 1012 M
E) 2.7 10-6 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molarity of an NaI solution that contains 5.3 g of NaI in 41.0 mL of solution?
A) 0.86 M
B) 0.035 M
C) 0.0077 M
D) 0.00027 M
E) 0.13 M
A) 0.86 M
B) 0.035 M
C) 0.0077 M
D) 0.00027 M
E) 0.13 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
A 1.185 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 2.264 g CO2 and 1.390 g H2O.What mass of oxygen is contained in the original sample?
A) 0.4117 g
B) 0.5673 g
C) 1.079 g
D) 0.8733 g
E) 0.2053 g
A) 0.4117 g
B) 0.5673 g
C) 1.079 g
D) 0.8733 g
E) 0.2053 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
Soft drink bottles are made of polyethylene terephthalate (PET),a polymer composed of carbon,hydrogen,and oxygen.If 1.9022 g PET is burned in oxygen it produces 0.6585 g H2O and 4.0216 g CO2.What is the empirical formula of PET?
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
In order to dilute 35.5 mL of 0.533 M HCl to 0.100 M,the volume of water that must be added is
A) 28.8 mL.
B) 6.66 mL.
C) 1.89 102 mL.
D) 1.50 10-3 mL.
E) 1.54 102 mL.
A) 28.8 mL.
B) 6.66 mL.
C) 1.89 102 mL.
D) 1.50 10-3 mL.
E) 1.54 102 mL.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
The combustion of 0.0272 mole of a hydrocarbon produces 1.9609 g H2O and 3.5927 g CO2.What is the molar mass of the hydrocarbon?
A) 16.0 g/mol
B) 30.1 g/mol
C) 44.1 g/mol
D) 72.2 g/mol
E) 92.1 g/mol
A) 16.0 g/mol
B) 30.1 g/mol
C) 44.1 g/mol
D) 72.2 g/mol
E) 92.1 g/mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
What volume of 0.464 M Na2CO3 solution contains 74.7 g of Na2CO3?
A) 0.327 L
B) 1.71 104 L
C) 1.52 L
D) 3.67 103 L
E) 0.658 L
A) 0.327 L
B) 1.71 104 L
C) 1.52 L
D) 3.67 103 L
E) 0.658 L

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
What mass of Na2CO3 is present in 0.250 L of a 0.852 M Na2CO3 solution?
A) 22.6 g
B) 26.5 g
C) 90.3 g
D) 31.1 g
E) 361 g
A) 22.6 g
B) 26.5 g
C) 90.3 g
D) 31.1 g
E) 361 g

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
The percent yield of a chemical reaction is calculated by dividing the actual yield by the ________ yield and multiplying by 100%.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
A 50.00-mL sample of a weak monoprotic acid is titrated with 0.0955 M NaOH.At the endpoint,it is found that 32.56 mL of titrant was used.What was the concentration of the weak acid?
A) 0.0622 M
B) 3.11 M
C) 0.0955 M
D) 5.87 10-5 M
E) 0.147 M
A) 0.0622 M
B) 3.11 M
C) 0.0955 M
D) 5.87 10-5 M
E) 0.147 M

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
A solution of sodium oxalate (Na2C2O4)in acidic solution is titrated with a solution of potassium permanganate (KMnO4)according to the following balanced chemical equation:
2KMnO4(aq)+ 8H2SO4(aq)+ 5Na2C2O4(aq) 2MnSO4(aq)+ 8H2O(l)+ 10CO2(g)+ 5Na2SO4(aq)+ K2SO4(aq)
What volume of 0.0388 M KMnO4 is required to titrate 0.134 g of Na2C2O4 dissolved in 20.0 mL of solution?
A) 1.38 mL
B) 3.45 mL
C) 10.3 mL
D) 25.8 mL
E) 20.0 mL
2KMnO4(aq)+ 8H2SO4(aq)+ 5Na2C2O4(aq) 2MnSO4(aq)+ 8H2O(l)+ 10CO2(g)+ 5Na2SO4(aq)+ K2SO4(aq)
What volume of 0.0388 M KMnO4 is required to titrate 0.134 g of Na2C2O4 dissolved in 20.0 mL of solution?
A) 1.38 mL
B) 3.45 mL
C) 10.3 mL
D) 25.8 mL
E) 20.0 mL

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
The numbers preceding the formulas in chemical equations are referred to as the ________ coefficients.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
If 0.1800 g of impure soda ash (Na2CO3)is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq)+ 2 HCl(aq) 2 NaCl(aq)+ H2O(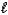 )+ CO2(g)
)+ CO2(g)
A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%
Na2CO3(aq)+ 2 HCl(aq) 2 NaCl(aq)+ H2O(
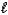 )+ CO2(g)
)+ CO2(g)A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
In combustion analysis,the gases produced by the combustion of a hydrocarbon are passed through a tube containing finely divided NaOH supported on asbestos.The purpose of the NaOH is to absorb ________ produced by the combustion reaction.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
An unknown diprotic acid (H2A)requires 44.39 mL of 0.111 M NaOH to completely neutralize a 0.580 g sample.Calculate the approximate molar mass of the acid.
A) 406 g/mol
B) 235 g/mol
C) 118 g/mol
D) 59 g/mol
E) 203 g/mol
A) 406 g/mol
B) 235 g/mol
C) 118 g/mol
D) 59 g/mol
E) 203 g/mol

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
The pH of purified water (or of a neutral solution)is _____.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
An impure sample of benzoic acid (C6H5COOH,122.12 g/mol)is titrated with 0.8067 M NaOH.A 5.109-g sample requires 36.97 mL of titrant to reach the endpoint.What is the percent by mass of benzoic acid in the sample?
C6H5COOH(aq)+ NaOH(aq) NaC6H5COO(aq)+ H2O(l)
A) 0.02442 %
B) 2.982 %
C) 100.0 %
D) 24.42 %
E) 71.29 %
C6H5COOH(aq)+ NaOH(aq) NaC6H5COO(aq)+ H2O(l)
A) 0.02442 %
B) 2.982 %
C) 100.0 %
D) 24.42 %
E) 71.29 %

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction of 5.07 g N2 with 0.722 g H2 produces 1.27 g NH3.The percent yield of this reaction is ________.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
PCl3 can be produced from the reaction of P4 with Cl2.
P4(s)+ 6 Cl2(g) 4 PCl3(g)
If 1.00 g P4 reacts with 1.00 g Cl2,which reactant is the limiting reactant? What mass of product may be produced?
P4(s)+ 6 Cl2(g) 4 PCl3(g)
If 1.00 g P4 reacts with 1.00 g Cl2,which reactant is the limiting reactant? What mass of product may be produced?

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is/are CORRECT?
1)Absorbance is directly proportional to the intensity of the incident light.
2)Absorbance is inversely proportional to the analyte concentration.
3)Absorbance is directly proportional to the path length of the light.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
1)Absorbance is directly proportional to the intensity of the incident light.
2)Absorbance is inversely proportional to the analyte concentration.
3)Absorbance is directly proportional to the path length of the light.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
The reaction of HCl with NaOH is represented by the equation
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
What volume of 0.686 M HCl is required to titrate 42.8 mL of 0.334 M NaOH?
A) 9.81 mL
B) 1.46 mL
C) 20.8 mL
D) 42.8 mL
E) 87.9 mL
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
What volume of 0.686 M HCl is required to titrate 42.8 mL of 0.334 M NaOH?
A) 9.81 mL
B) 1.46 mL
C) 20.8 mL
D) 42.8 mL
E) 87.9 mL

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
The following equation: A = 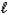
C (where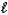
is path length and C is concentration)is known as the _____-Lambert law.
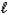
C (where
is path length and C is concentration)is known as the _____-Lambert law.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
Transmittance,T,is defined as the intensity of light transmitted through a sample divided by the intensity of light incident on the sample.The absorbance,A,is defined as
A) A = -log T
B) A = log (-T)
C) A = 10T
D) A = 10-T
E) A = 10-1/T
A) A = -log T
B) A = log (-T)
C) A = 10T
D) A = 10-T
E) A = 10-1/T

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
Calibration of a spectrophotmeter using a series of green dye containing solutions provides a set of data which follows the Beer-Lambert Law,with a trendline of y = 1.070 103x (y-axis = absorbance,x-axis = molar concentration).What is the molar concentration of a solution with an absorbance of 0.3131?
A) 0.2926 M
B) 0.3131 M
C) 3.194 M
D) 0.9346 M
E) 0.6869
A) 0.2926 M
B) 0.3131 M
C) 3.194 M
D) 0.9346 M
E) 0.6869

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck


