Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
What mass of iron can be produced from the reaction of 175 kg Fe2O3 with 385 kg CO?
Fe2O3(s)+ 3 CO(g) 2 Fe(s)+ 3 CO2(g)
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
D
What volume of 0.464 M Na2CO3 solution contains 74.7 g of Na2CO3?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
A solution of sodium oxalate (Na2C2O4)in acidic solution is titrated with a solution of potassium permanganate (KMnO4)according to the following balanced chemical equation:
2KMnO4(aq)+ 8H2SO4(aq)+ 5Na2C2O4(aq) 2MnSO4(aq)+ 8H2O(l)+ 10CO2(g)+ 5Na2SO4(aq)+ K2SO4(aq)
What volume of 0.0388 M KMnO4 is required to titrate 0.134 g of Na2C2O4 dissolved in 20.0 mL of solution?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
C
The compound P4S3 is used in matches.It reacts with oxygen to produce P4O10 and SO2.The chemical equation is shown below.
P4S3(s)+ O2(g) P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
(Multiple Choice)
4.9/5  (47)
(47)
Nitric oxide,NO,is made from the oxidation of NH3 as follows:
4NH3 + 5O2 4NO + 6H2O
If 9.4-g of NH3 gives 12.0 g of NO,what is the percent yield of NO?
(Multiple Choice)
4.7/5  (29)
(29)
The reaction of 11.9 g of CHCl3 with excess chlorine produced 10.3 g of CCl4,carbon tetrachloride:
2CHCl3 + 2Cl2 2CCl4 + 2HCl
What is the percent yield?
(Multiple Choice)
4.9/5  (33)
(33)
The numbers preceding the formulas in chemical equations are referred to as the ________ coefficients.
(Short Answer)
4.8/5  (34)
(34)
In order to dilute 35.5 mL of 0.533 M HCl to 0.100 M,the volume of water that must be added is
(Multiple Choice)
4.7/5  (35)
(35)
An unknown diprotic acid (H2A)requires 44.39 mL of 0.111 M NaOH to completely neutralize a 0.580 g sample.Calculate the approximate molar mass of the acid.
(Multiple Choice)
4.8/5  (37)
(37)
Complete combustion of a 0.30-mol sample of a hydrocarbon,CxHy,gives 1.80 mol of CO2 and 1.20 mol of H2O.The molecular formula of the original hydrocarbon is
(Multiple Choice)
4.8/5  (38)
(38)
Under certain conditions the reaction of ammonia with excess oxygen will produce a 29.5% yield of NO.What mass of NH3 must react with excess oxygen to yield 157 g NO?
4 NH3(g)+ 5 O2(g) 4 NO(g)+ 6 H2O(g)
(Multiple Choice)
4.8/5  (42)
(42)
Transmittance,T,is defined as the intensity of light transmitted through a sample divided by the intensity of light incident on the sample.The absorbance,A,is defined as
(Multiple Choice)
4.9/5  (32)
(32)
What volume of 2.52 M HCl is required to prepare 176.5 mL of 0.449 M HCl?
(Multiple Choice)
4.9/5  (39)
(39)
Aspirin (C9H8O4)is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3( 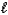 ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 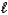 )
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
(Multiple Choice)
4.8/5  (39)
(39)
Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride
(M = 102.1 g/mol).C7H6O3(s)+ C4H6O3( 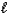 ) C9H8O4(s)+ C2H4O2(
) C9H8O4(s)+ C2H4O2( 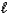 )
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
(Multiple Choice)
4.9/5  (35)
(35)
Magnesium reacts with iodine gas at high temperatures to form magnesium iodide.What mass of MgI2 can be produced from the reaction of 5.15 g Mg and 50.0 g I2?
(Multiple Choice)
4.9/5  (49)
(49)
Sulfur dioxide will react with water to form sulfurous acid (see balanced equation below).
SO2(g)+ H2O(l) H2SO3(l)
What mass of sulfur dioxide is needed to prepare 21.06 g of H2SO3(l)?
(Multiple Choice)
4.8/5  (36)
(36)
The percent yield of a chemical reaction is calculated by dividing the actual yield by the ________ yield and multiplying by 100%.
(Short Answer)
4.8/5  (36)
(36)
Hydrogen peroxide decomposes into oxygen and water.What mass of oxygen is formed from the decomposition of 125 g of H2O2?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)