Deck 1: Atomic and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 1: Atomic and Molecular Structure
1
An atom of which element would have an electron configuration of 1s22s22p63s23p1?
A)Al
B)Ne
C)B
D)Si
E)Na
A)Al
B)Ne
C)B
D)Si
E)Na
Al
2
Identify which carbon atom in the molecule below is most oxidized. 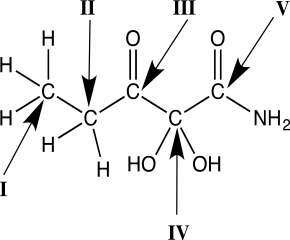
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
V
3
Which electron configuration is correct for the carbon of a carbocation?
A)1s22s22p1
B)1s22s22p3
C)1s22s22p5
D)1s22s22p63s23p5
E)1s22s22p4
A)1s22s22p1
B)1s22s22p3
C)1s22s22p5
D)1s22s22p63s23p5
E)1s22s22p4
1s22s22p1
4
Which of the following is an example of an electrostatic attractive force between particles at the atomic level?
A)Neutrons attract protons.
B)Protons repel protons.
C)Core electrons attract valence electrons.
D)Protons attract electrons.
E)Electrons attract neutrons.
A)Neutrons attract protons.
B)Protons repel protons.
C)Core electrons attract valence electrons.
D)Protons attract electrons.
E)Electrons attract neutrons.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
Which point on the following diagram can be extrapolated to identify the length and strength of a chemical bond? 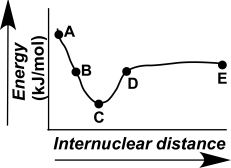
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
A C-O single bond is 143 pm in length,whereas an O-O single bond is 148 pm in length. Which bond is weaker and why?
A)The C-O bond is weaker because O is more electronegative than C.
B)The C-O bond is weaker because each O atom is electronegative and pulls the shared electrons toward itself.
C)The O-O bond is weaker because oxygen has d orbitals to engage in bonding.
D)The C-O bond is weaker because C is more electronegative than O.
E)The O-O bond is weaker because both oxygens are equally electronegative and pull the shared electrons toward themselves.
A)The C-O bond is weaker because O is more electronegative than C.
B)The C-O bond is weaker because each O atom is electronegative and pulls the shared electrons toward itself.
C)The O-O bond is weaker because oxygen has d orbitals to engage in bonding.
D)The C-O bond is weaker because C is more electronegative than O.
E)The O-O bond is weaker because both oxygens are equally electronegative and pull the shared electrons toward themselves.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
Identify which carbon atom in the molecule below is most reduced. 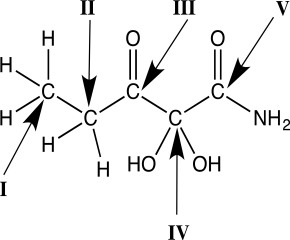
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
Which line structure is correct for Molecule Z? 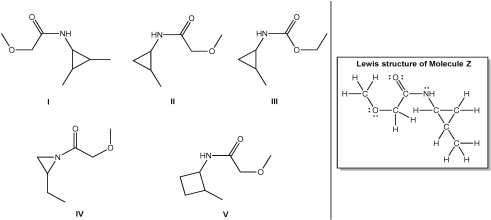
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the interesting structure below,called a dibromocarbene.The carbon of the dibromocarbene has one lone electron pair and two separate covalent bonds to individual bromine atoms.What is the formal charge on the carbon atom of the dibromocarbene? 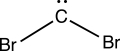
A)+2
B)+1
C)0
D)-1
E)-2

A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
Which electron configuration is correct for a carbon atom with a formal charge of -1?
A)1s22s22p63s1
B)1s22s22p3
C)1s22s22p5
D)1s22s22p63s23p5
E)1s22s22p4
A)1s22s22p63s1
B)1s22s22p3
C)1s22s22p5
D)1s22s22p63s23p5
E)1s22s22p4

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
Evaluate the Lewis structure below and determine its legitimacy. 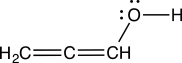
A)The structure is legitimate.
B)The structure is not legitimate, because the oxygen does not have an octet.
C)The structure is not legitimate, because the formal charges are not shown.
D)The structure is not legitimate, because the middle carbon lacks an octet.
E)The structure is not legitimate, because the leftmost carbon is missing a lone pair.
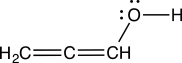
A)The structure is legitimate.
B)The structure is not legitimate, because the oxygen does not have an octet.
C)The structure is not legitimate, because the formal charges are not shown.
D)The structure is not legitimate, because the middle carbon lacks an octet.
E)The structure is not legitimate, because the leftmost carbon is missing a lone pair.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
Which orbital does not house core electrons for a bromine atom?
A)1s
B)4p
C)3p
D)2s
E)3s
A)1s
B)4p
C)3p
D)2s
E)3s

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
Which point on the following diagram represents two atoms functioning independently? 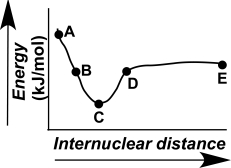
)
A)A
B)B
C)C
D)D
E)E

)
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
When two Lewis structures are related as resonance forms,which of the following are true?
I. When compared,the resonance forms have the same atoms connected in the same order.
II. Either individual Lewis structure can be used as an accurate representation of valence electron distribution.
III.Electrons in single bonds may be delocalized in the resonance forms.
IV.Electrons in a multiple bond may be delocalized in the resonance forms.
V. A lone pair of electrons on an atom adjacent to a multiple bond can be delocalized in the resonance forms.
A)All are true.
B)Only I, II, IV, and V are true.
C)Only I, III, IV, and V are true.
D)Only I, IV, and V are true.
E)Only I and IV are true.
I. When compared,the resonance forms have the same atoms connected in the same order.
II. Either individual Lewis structure can be used as an accurate representation of valence electron distribution.
III.Electrons in single bonds may be delocalized in the resonance forms.
IV.Electrons in a multiple bond may be delocalized in the resonance forms.
V. A lone pair of electrons on an atom adjacent to a multiple bond can be delocalized in the resonance forms.
A)All are true.
B)Only I, II, IV, and V are true.
C)Only I, III, IV, and V are true.
D)Only I, IV, and V are true.
E)Only I and IV are true.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
What is the molecular formula of this compound? 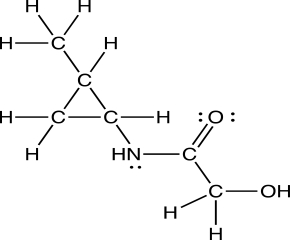
A)C6H12NO2
B)C5H12NO2
C)C5H11NO2
D)C6H11NO2
E)C6H13NO2

A)C6H12NO2
B)C5H12NO2
C)C5H11NO2
D)C6H11NO2
E)C6H13NO2

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
Which carbon atom has an oxidation state of +3? 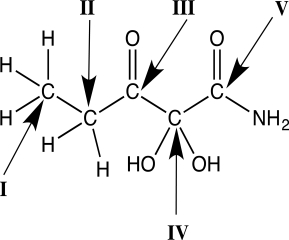
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Which electron configuration is correct for a Ca2+ ion?
A)1s22s22p63s23p1
B)1s22s22p63s23p64s2
C)1s22s22p63s23p6
D)1s22s22p63s23p64s24p6
E)1s22s22p63s2
A)1s22s22p63s23p1
B)1s22s22p63s23p64s2
C)1s22s22p63s23p6
D)1s22s22p63s23p64s24p6
E)1s22s22p63s2

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
How many valence electrons are assigned to oxygen when determining formal charge in the ionic compound sodium methoxide,NaOCH3?
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following Lewis structures violates the octet rule and is therefore incorrect? 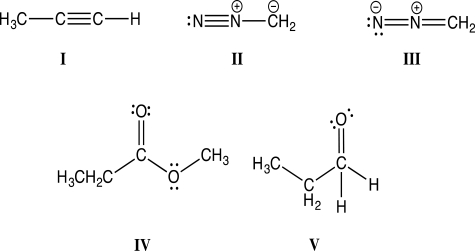
A)Structure I
B)Structure II
C)Structure III
D)Structure IV
E)Structure V

A)Structure I
B)Structure II
C)Structure III
D)Structure IV
E)Structure V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
How many total valence electrons are used in the structure of ammonium chloride,NH4Cl?
A)13
B)14
C)15
D)16
E)28
A)13
B)14
C)15
D)16
E)28

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
Which condensed formula contains an ester?
A)(CH3CH2)2O
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
A)(CH3CH2)2O
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
A compound with a molecular formula of C7H7Br has a melting point of 203°C.The compound is soluble in water but not in diethyl ether.Based on your knowledge of organic structure,is the compound most stable in its ionic or covalent form? Justify your answer. 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
Which condensed formula contains a carboxylic acid?
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
Compare Structure A with Structures B,C,and D.Is Structure B,C,or D a resonance structure of A? Justify your response.For any structures that are resonance forms,use curved arrows to show how the resonance forms are interconverted. 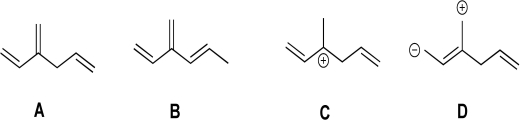


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
Predict which carbon atom should have the greatest negative charge density.Explain your answer.What would the electrostatic potential map show for this carbon,in comparison with others in the molecule? 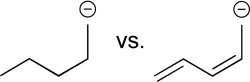


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following amino acids possesses two hydrogen atoms adjacent to the carboxylic acid?
A)Serine
B)Phenylalanine
C)Glycine
D)Tryptophan
E)Lysine
A)Serine
B)Phenylalanine
C)Glycine
D)Tryptophan
E)Lysine

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
Using a chemical reaction to convert an alcohol to an oxonium species is a highly valued tool in every organic chemist's arsenal (see the figure below).Although this reaction was not covered in the current chapter,it will be discussed in due course.Reconsider your response to the preceding question.Can you identify the two different atoms of the oxonium species to which a negatively charged species might be most attracted? Explain.Hint: It is not the oxygen with the positive charge. 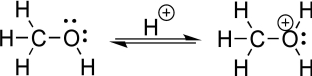


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
Consider two solvents that are commonly used for organic chemistry reactions: CH2Cl2 and CCl4.Interestingly,studies have shown that one of these solvents is polar and one is nonpolar.Draw valid Lewis structures for these two molecules.Show bond dipoles and both partial charges (use δ+ and δ-).How would the electrostatic potential maps for the two molecules be similar? How would they be different?

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
Which condensed formula contains a ketone?
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
Which individual structures below could be contributing resonance structures to the given hybrid structure? 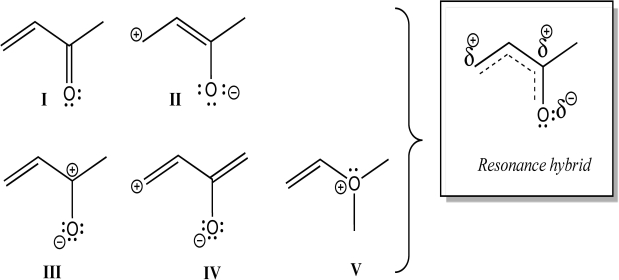
A)All are contributing structures.
B)All but V are contributing structures.
C)I, II, and III are contributing structures.
D)I, III, and IV are contributing structures.
E)Only I and III are contributing structures.

A)All are contributing structures.
B)All but V are contributing structures.
C)I, II, and III are contributing structures.
D)I, III, and IV are contributing structures.
E)Only I and III are contributing structures.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
Which would you expect to be a stronger bond: C-Si or C-C?

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here. 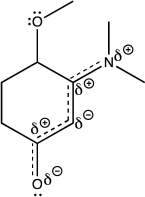


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
Oxygen is an important heteroatom found in many organic molecules.Consider methanol and its protonated derivative,shown below.Indicate relevant bond dipoles using dipole arrows.How does an oxygen with a positive charge,called an oxonium species,influence the magnitude of the partial positive charge on the carbon atom? Which oxygen-carbon bond do you think is more difficult to break? Explain. 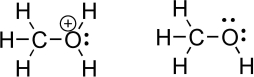
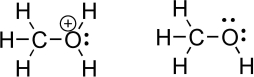

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
Using line structures,draw the individual resonance contributors from the resonance hybrid structure given here. 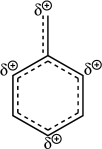


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
Draw a Lewis structure of thionyl chloride,SOCl2,showing all lone pairs.Show each bond dipole using a dipole arrow.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
Naltrexone is an FDA-approved treatment for alcoholism that targets the mu opioid receptor.Name four functional groups that are present in naltrexone. 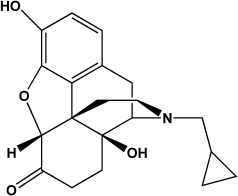
A)Amine, phenol, aldehyde, ether
B)Amine, phenol, amide, alcohol
C)Amine, phenol, ketone, alcohol
D)Ether, ketone, amide, alcohol
E)Ketone, phenol, alcohol, ester

A)Amine, phenol, aldehyde, ether
B)Amine, phenol, amide, alcohol
C)Amine, phenol, ketone, alcohol
D)Ether, ketone, amide, alcohol
E)Ketone, phenol, alcohol, ester

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
For which of the following amino acids can resonance forms be drawn for its side chain?
A)Alanine
B)Methionine
C)Glycine
D)Proline
E)Histidine
A)Alanine
B)Methionine
C)Glycine
D)Proline
E)Histidine

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
Which condensed formula contains an aldehyde functional group?
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH
A)CH3COCH3
B)CH3CO2CH2CH3
C)CH3CH2CH2COOH
D)CH3CH2OH
E)CH3COH

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
The evolution of a chemical bond can be tracked by plotting energy versus internuclear distance,as shown in the figure here.Describe what is occurring at the atomic level for points A-E on the graph. 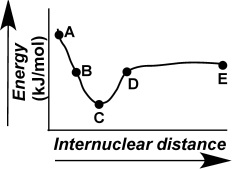


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
In sum,how many total hydrogen atoms are directly connected to the phenyl rings found in the molecule below? 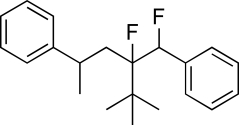
A)8
B)9
C)10
D)11
E)12

A)8
B)9
C)10
D)11
E)12

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the molecules below as monosaccharides,disaccharides,or carbohydrates.Explain the distinction between these classes of biologically active organic molecules. 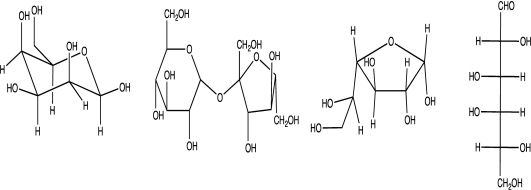


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
A carbonyl,the C 
O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer.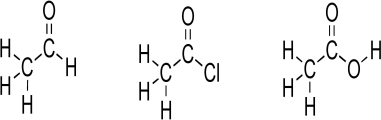

O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer.


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
Are the Lewis structures for sulfuric acid shown below related as resonance structures? Explain. 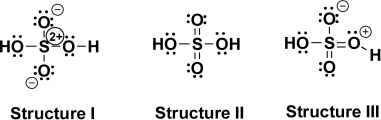


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
(a)Sulfuric acid,H2SO4,is an important strong oxo acid in organic chemistry.Propose Lewis structures for sulfuric acid,showing all lone pairs and formal charges,using the following guidelines:
In Structure I,one atom has a +2 formal charge.Two other atoms each possess a charge of -1.All atoms in Structure II are completely neutral.In Structure III,one atom has a +1 charge and another a -1 charge.
(b)Which Lewis structure is most stable? Why?
The neutral structure is most stable.The number of covalent bonds is maximized,and charges are minimized (e.g.,there are no individual formal charges on atoms).
In Structure I,one atom has a +2 formal charge.Two other atoms each possess a charge of -1.All atoms in Structure II are completely neutral.In Structure III,one atom has a +1 charge and another a -1 charge.
(b)Which Lewis structure is most stable? Why?
The neutral structure is most stable.The number of covalent bonds is maximized,and charges are minimized (e.g.,there are no individual formal charges on atoms).

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
Draw all possible resonance forms for anisole using appropriate arrow notation.Which resonance structure is most stable? Least stable? Draw the resonance hybrid for anisole,indicating all partial charges. 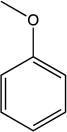


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
The six unique hydrogen atoms in the molecule below are labeled a-f.Suppose we individually replace each of these unique hydrogen atoms with a hydroxyl group (-OH)and draw a new molecule of formula C6H10O2.(In the cases of Ha,Hb,and Hc,substitute only one designated H with an OH.)Which of the new -OH groups would have localized lone pairs on oxygen? Which of the -OH groups would have a delocalized lone pair? Finally,for each molecule,identify the new functional group created. 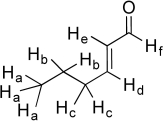


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
Global warming and ozone depletion both have far reaching environmental consequences and are linked by human cause.Propose two reasonable structures of ozone,O3,that differ in electron delocalization and do not contain a ring.Show how these two structures are interconverted using appropriate arrow notation.Explain the relationship between these molecules.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
Consider a peptide bond formed from the amino acids alanine and serine.Redraw the dipeptide from the figure below using a line structure.Once this structure is drawn,place a box around the amide bond.Circle the parts of the dipeptide that originate from serine and alanine. 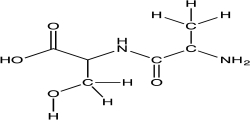


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
To which carbon atoms in anisole would a positively charged species,called "E+," bond? Explain your answer using the concept of resonance.HINT: Refer to your response from 16 for further insight. 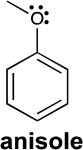


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
Peptide bonds are the building blocks of proteins.Consider a peptide bond formed from the amino acids alanine and serine,shown below.Draw the resonance forms and the resonance hybrid for the amide bond of the dipeptide.Use appropriate arrow notation. 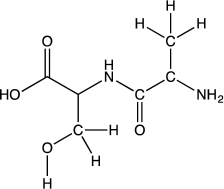


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the functional groups present in the structure of the dipeptide shown below.In your answer,do not use "alkane." 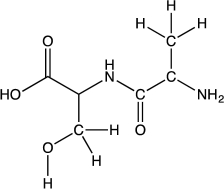


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
Amino acids are important building blocks in chemistry.Consider the structure below,which can be formed from reaction of an amino acid with another compound.Identify the amino acid that is used in the synthesis.Clearly label the amino acid side chain,the alpha carbon,the amine,and the carboxylic acid within the larger structure.What parts of the larger molecule do not come from the amino acid? 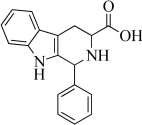


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
The way atoms are connected to each other in an organic structure determines the chemical behavior of the structure.Using line structures,propose individual molecules with the formula C4H8O that contain the following functional groups:
(a)A ketone (e)A cyclic ether that is not an epoxide
(b)An aldehyde with a branched alkane (f)An epoxide
(c)An aldehyde with an unbranched alkane (g)An acyclic ether
(d)A cycloalkane (h)An alkene and an alcohol
(a)A ketone (e)A cyclic ether that is not an epoxide
(b)An aldehyde with a branched alkane (f)An epoxide
(c)An aldehyde with an unbranched alkane (g)An acyclic ether
(d)A cycloalkane (h)An alkene and an alcohol

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
Tubulysin D is a potent cytotoxic compound that interferes with mitosis.Identify the following structural features of Tubulysin D.
(a)Place a box around any amide found in the molecule.
(b)Circle the phenyl group(s).
(c)Place a triangle around the carboxylic acid(s).
(d)Star any carbon atoms with a -3 oxidation state.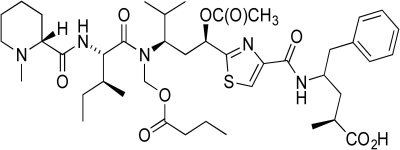
(a)Place a box around any amide found in the molecule.
(b)Circle the phenyl group(s).
(c)Place a triangle around the carboxylic acid(s).
(d)Star any carbon atoms with a -3 oxidation state.


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


