Exam 1: Atomic and Molecular Structure
Naltrexone is an FDA-approved treatment for alcoholism that targets the mu opioid receptor.Name four functional groups that are present in naltrexone. 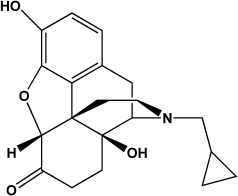
C
In sum,how many total hydrogen atoms are directly connected to the phenyl rings found in the molecule below? 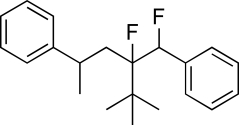
C
A carbonyl,the C  O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer.
O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer. 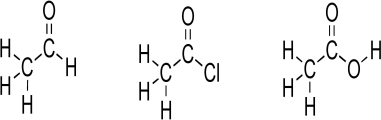
The point of difference in the three structures is the substitution on the carbon of the carbonyl.Any property that influences electron distribution will influence the magnitude of the positive charge.Effects are either electron withdrawing (e.g.,an electronegative atom)or electron donating (e.g.,compare the Pauling electronegativity values and resonance potential of each atom).The carbonyl carbon of the acyl chloride has the greatest partial positive charge.There are three lone pairs on Cl and two on the O of the OH.Because one of the oxygen lone pairs can be delocalized through resonance,electron density is added to the C  O,and the partial positive charge of the carbonyl carbon is reduced.Given that Cl does not typically engage in resonance,then the electron withdrawing nature of the electronegative Cl predominates.
O,and the partial positive charge of the carbonyl carbon is reduced.Given that Cl does not typically engage in resonance,then the electron withdrawing nature of the electronegative Cl predominates. 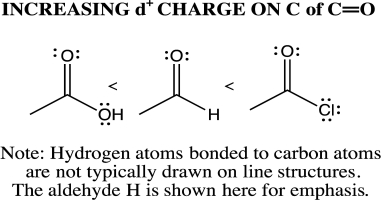
A C-O single bond is 143 pm in length,whereas an O-O single bond is 148 pm in length. Which bond is weaker and why?
Tubulysin D is a potent cytotoxic compound that interferes with mitosis.Identify the following structural features of Tubulysin D.
(a)Place a box around any amide found in the molecule.
(b)Circle the phenyl group(s).
(c)Place a triangle around the carboxylic acid(s).
(d)Star any carbon atoms with a -3 oxidation state. 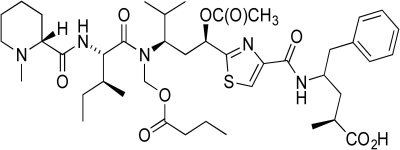
Identify which carbon atom in the molecule below is most oxidized. 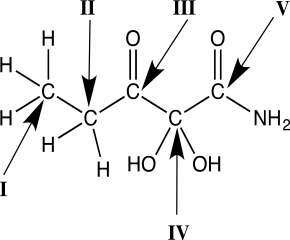
Consider the interesting structure below,called a dibromocarbene.The carbon of the dibromocarbene has one lone electron pair and two separate covalent bonds to individual bromine atoms.What is the formal charge on the carbon atom of the dibromocarbene? 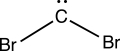
To which carbon atoms in anisole would a positively charged species,called "E+," bond? Explain your answer using the concept of resonance.HINT: Refer to your response from 16 for further insight. 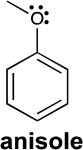
The way atoms are connected to each other in an organic structure determines the chemical behavior of the structure.Using line structures,propose individual molecules with the formula C4H8O that contain the following functional groups:
(a)A ketone (e)A cyclic ether that is not an epoxide
(b)An aldehyde with a branched alkane (f)An epoxide
(c)An aldehyde with an unbranched alkane (g)An acyclic ether
(d)A cycloalkane (h)An alkene and an alcohol
Which electron configuration is correct for the carbon of a carbocation?
Which of the following is an example of an electrostatic attractive force between particles at the atomic level?
Consider two solvents that are commonly used for organic chemistry reactions: CH2Cl2 and CCl4.Interestingly,studies have shown that one of these solvents is polar and one is nonpolar.Draw valid Lewis structures for these two molecules.Show bond dipoles and both partial charges (use δ+ and δ-).How would the electrostatic potential maps for the two molecules be similar? How would they be different?
Which point on the following diagram represents two atoms functioning independently? 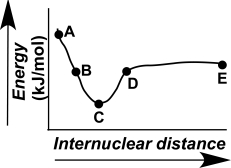 )
)
Draw a Lewis structure of thionyl chloride,SOCl2,showing all lone pairs.Show each bond dipole using a dipole arrow.
How many valence electrons are assigned to oxygen when determining formal charge in the ionic compound sodium methoxide,NaOCH3?
Predict which carbon atom should have the greatest negative charge density.Explain your answer.What would the electrostatic potential map show for this carbon,in comparison with others in the molecule? 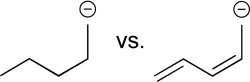
A compound with a molecular formula of C7H7Br has a melting point of 203°C.The compound is soluble in water but not in diethyl ether.Based on your knowledge of organic structure,is the compound most stable in its ionic or covalent form? Justify your answer. 
For which of the following amino acids can resonance forms be drawn for its side chain?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)