Deck 6: Isomerism 1: Conformational and Constitutional Isomers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 6: Isomerism 1: Conformational and Constitutional Isomers
1
Rank the steric strain of the following types of conformations from highest energy to lowest energy.
A)Anti > eclipsed > gauche
B)Eclipsed > anti > gauche
C)Gauche > anti > eclipsed
D)Eclipsed > gauche > anti
A)Anti > eclipsed > gauche
B)Eclipsed > anti > gauche
C)Gauche > anti > eclipsed
D)Eclipsed > gauche > anti
Eclipsed > gauche > anti
2
Which of the following compounds has/have substituents cis to each other? 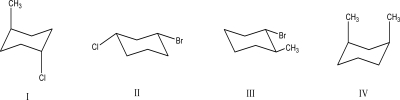
A)I
B)II
C)III
D)IV
E)II and IV

A)I
B)II
C)III
D)IV
E)II and IV
II and IV
3
How many fluorine atoms are in equatorial positions in the following chair structure? 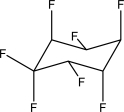
A)One
B)Two
C)Three
D)Four
E)Five

A)One
B)Two
C)Three
D)Four
E)Five
Three
4
Which of the following dash-wedge structures best represents the Newman projection below? 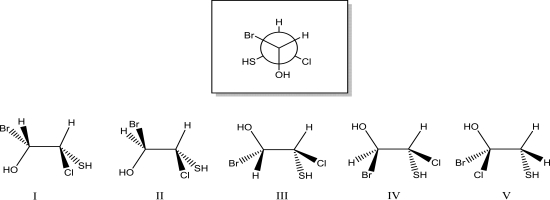
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the least stable cyclohexane conformation?
A)Chair
B)Half-chair
C)Twist-boat
D)Boat
E)Crown
A)Chair
B)Half-chair
C)Twist-boat
D)Boat
E)Crown

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following dash-wedge structures best represents the Newman projection below? 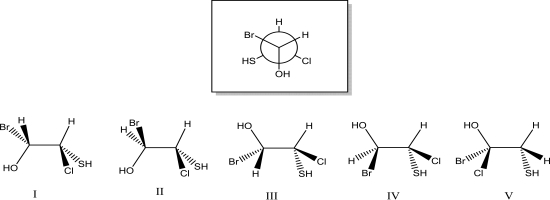
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following molecules has the largest molar heat of combustion? 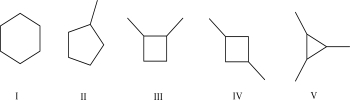
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Which substituents are gauche to each other? 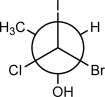
A)I and Br
B)I and OH
C)CH3 and Br
D)H and I
E)Cl and Br

A)I and Br
B)I and OH
C)CH3 and Br
D)H and I
E)Cl and Br

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
The Newman projection looking down the indicated bond in the species below is best represented by which of the following (I,II,III,IV,or V)? 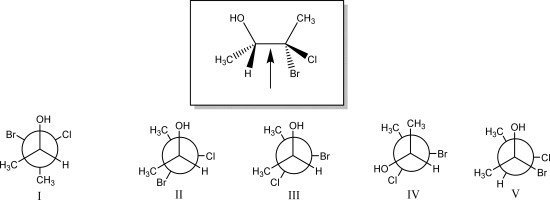
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
The Newman projection looking down the indicated bond in the species below is best represented by which of the following (I,II,III,IV,or V)? 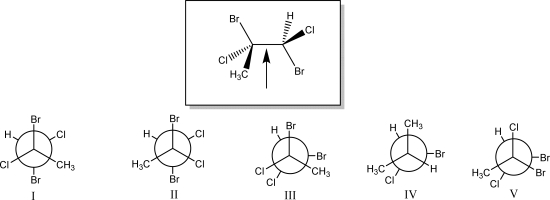
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Which conformer is highest in energy? 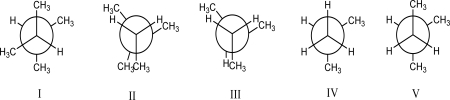
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which Newman projection results from rotating the front carbon 240° clockwise? 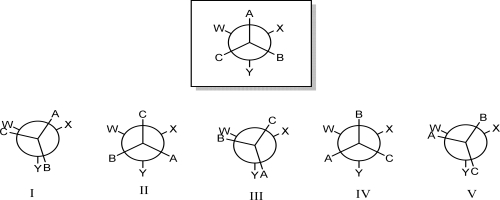
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following pairs do not have the same connectivity?
A)Two stereoisomers
B)Two conformational isomers
C)Cyclohexane chair conformations before and after a chair flip
D)Two constitutional isomers
E)Two Newman projection rotamers
A)Two stereoisomers
B)Two conformational isomers
C)Cyclohexane chair conformations before and after a chair flip
D)Two constitutional isomers
E)Two Newman projection rotamers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following has the least ring strain? 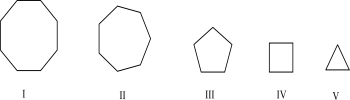
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which substituents are anti to each other? 
A)OCH3 and H
B)Cl and CH2CH3
C)Br and SH
D)H and Cl
E)CH2CH3 and Br

A)OCH3 and H
B)Cl and CH2CH3
C)Br and SH
D)H and Cl
E)CH2CH3 and Br

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following types of isomer differs only by the rotation about single bonds?
A)Constitutional
B)Configurational
C)Conformational
D)Diastereomers
E)Enantiomers
A)Constitutional
B)Configurational
C)Conformational
D)Diastereomers
E)Enantiomers

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following Newman projections is least stable? 
A)I
B)II
C)III
D)IV
E)All are equally stable.

A)I
B)II
C)III
D)IV
E)All are equally stable.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
How many degrees does the front carbon have to be rotated clockwise to result in a gauche conformer? 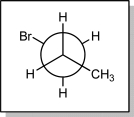
A)60°
B)90°
C)109.5°
D)120°
E)300°

A)60°
B)90°
C)109.5°
D)120°
E)300°

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Which structure best represents the following disubstituted cyclohexane? 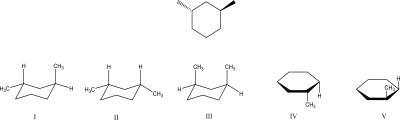
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Which molecule represents the chair-flipped conformer of the given compound? 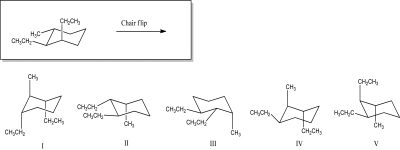
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the index of hydrogen deficiency for the following formula: C8H12NOF2-.
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Which set of molecules does not consist of constitutional isomers? 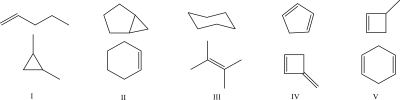
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following isomers is most stable? 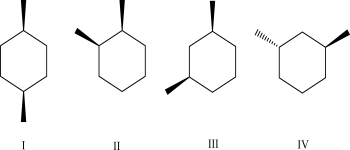
A)I
B)II
C)III
D)IV
E)I, II, and III have the same stability and are more stable than IV.

A)I
B)II
C)III
D)IV
E)I, II, and III have the same stability and are more stable than IV.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Which is the most stable conformation of the given trisubstituted cyclohexane below? 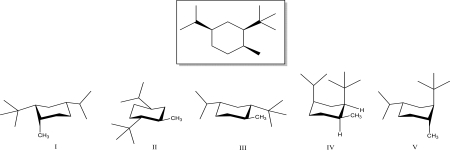
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Which set of molecules are not considered constitutional isomers? 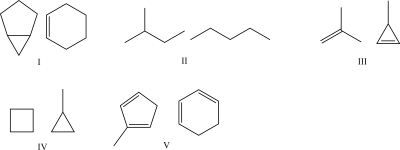
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Perform a conformational analysis of 1,1-dichloropropane looking down the C1C2 bond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a constitutional isomer for the following compound. 


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the least stable Newman projection for 1-iodobutane looking down the C1C2 bond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Conformational isomers differ by __________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the most stable chair conformation of the following Haworth projection? 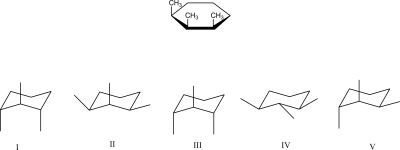
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Perform a conformational analysis of bromoethane looking down the C1C2 bond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the dash-wedge structure that corresponds to the following Newman projection. 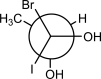


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the dash-wedge structure that corresponds to the following Newman projection. 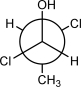


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Which Haworth projection corresponds to the following chair conformation? 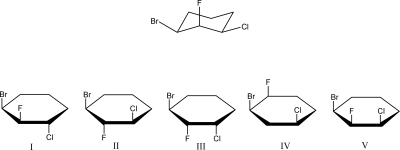
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following chair conformations is lowest in energy? 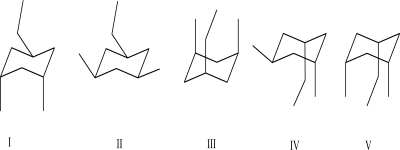
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules has the highest IHD? 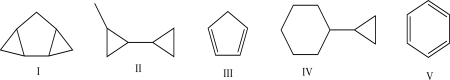
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the Newman projection that corresponds to the following species. 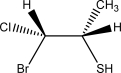


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the Newman projection for the following species. 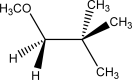


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
What is the most stable chair conformation for the following molecule? 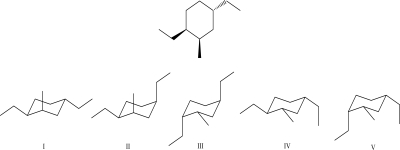
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the most stable Newman projection for 1-bromo-2-chloroethane.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the IHD for the formula C7H11BrO.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the following molecule after it undergoes a chair flip. 


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Draw six constitutional isomers for C4H11N.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the most stable chair conformation for the following molecule. 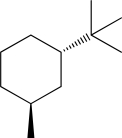


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Draw a Haworth projection for the following trisubstituted cyclohexane 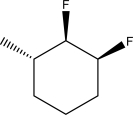


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Clearly draw the following molecule with all of its substituents in axial positions. 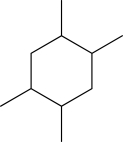


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Explain why cyclohexane has less ring strain than cyclopropane.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the most Newman projection for 1-iodobutane looking down the C1-C2 bond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
How many of the following chair conformations have CH3 groups that are trans to each other? 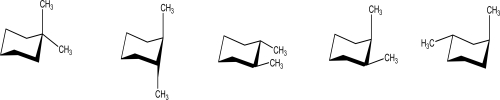


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a chair structure for the following Haworth structure. 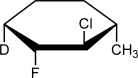


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Would the cis or trans isomer be more stable for the following disubstituted cyclohexane? Explain. 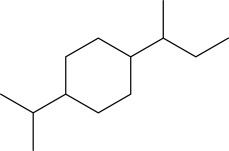


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Clearly draw the most stable conformer of the following molecule in chair formation. 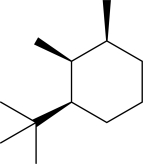


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
A molecule has two rings,three double bonds,and two triple bonds.How many IHD units does this molecule have?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
What is the IHD for the formula C8H17NOClF?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
How many CH3 CH3 gauche interactions are present in the following Newman projection? 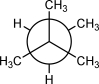


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Label each of the following molecules as cis or trans. 


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Draw all alkyne-containing isomers of C5H8.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Give five constitutional isomers for the formula C4H6.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Would the cis or trans isomer be more stable for the following disubstituted cyclohexane? Explain. 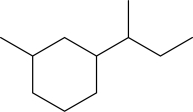


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the most stable chair conformation for the following compound. 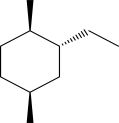


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


