Deck 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles
1
Which of the following solvents would be the best choice to use for the reduction of a ketone using LiAlH4?
A)Isopropanol
B)Water
C)Acetic acid
D)Diethyl ether
E)Ethanol
A)Isopropanol
B)Water
C)Acetic acid
D)Diethyl ether
E)Ethanol
Diethyl ether
2
Predict the product of the following reaction. 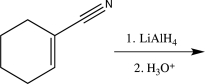
A)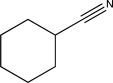
B)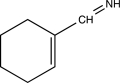
C)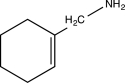
D)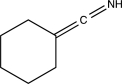
E)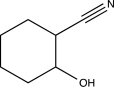

A)

B)

C)

D)

E)


3
Which of the following compounds would you expect to react with a nucleophile at the lowest rate? 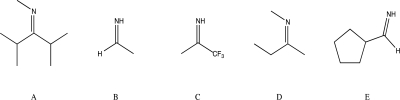
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E
A
4
Which of the following correctly explains why LiAlH4 is a stronger reducing agent than NaBH4?
A)LiAlH4 has less steric hindrance than NaBH4.
B)The electronegativity of Al is lower than that of B.
C)The electronegativity of Li is higher than that of Na.
D)Hydrogen has a greater partial positive charge in LiAlH4 than in NaBH4.
E)The electronegativity difference between Li and H is greater than the difference between Na and H.
A)LiAlH4 has less steric hindrance than NaBH4.
B)The electronegativity of Al is lower than that of B.
C)The electronegativity of Li is higher than that of Na.
D)Hydrogen has a greater partial positive charge in LiAlH4 than in NaBH4.
E)The electronegativity difference between Li and H is greater than the difference between Na and H.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Predict the major product of the following synthetic scheme. 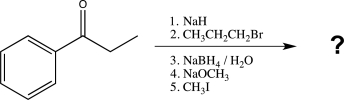
A)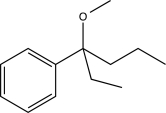
B)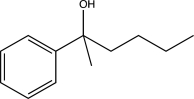
C)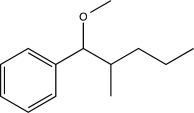
D)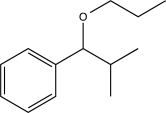
E)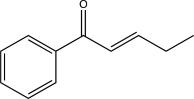

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following structures does not contain an ylide?
A)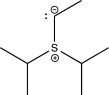
B)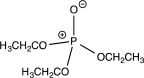
C)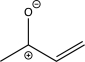
D)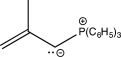
E)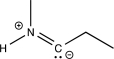
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Choose an appropriate set of reagents to carry out the synthetic step shown below. 
A)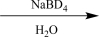
B)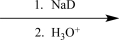
C)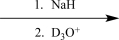
D)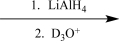
E)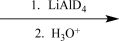

A)
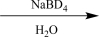
B)
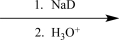
C)
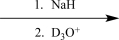
D)
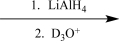
E)
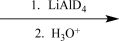

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following nucleophiles would tend to favor conjugate addition to an α,β-unsaturated carbonyl?
A)CH3CH2O
B)LiAlH4
C)CH3(CH2)3MgBr
D)CH3Li
E)(C6H5)3P- CH2
A)CH3CH2O
B)LiAlH4
C)CH3(CH2)3MgBr
D)CH3Li
E)(C6H5)3P- CH2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
The chemical behavior of 13C is essentially identical to that of 12C.If the reaction below is carried out,what percentage of the carbon atoms in the product will be 13C? 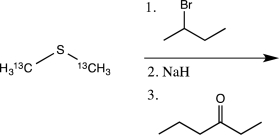
A)0 percent
B)10 percent
C)20 percent
D)30 percent
E)50 percent

A)0 percent
B)10 percent
C)20 percent
D)30 percent
E)50 percent

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
What is the major driving force for the mechanistic step shown below? 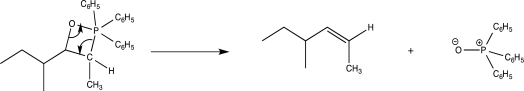
A)Stability of C C bond
C bond
B)Completing the octet around P
C)Electron rich to electron poor
D)Formation of strong P+O bond
E)Release of ring strain

A)Stability of C
 C bond
C bondB)Completing the octet around P
C)Electron rich to electron poor
D)Formation of strong P+O bond
E)Release of ring strain

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following reactions would not result in the formation of a racemic mixture of products?
A)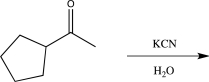
B)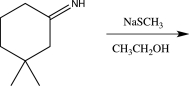
C)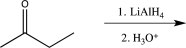
D)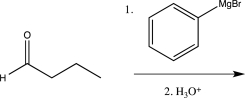
E)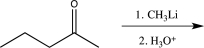
A)

B)

C)
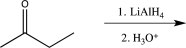
D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
With respect to direct and conjugate addition,which of the following statements is false?
A)Reversible addition of a nucleophile generally favors conjugate addition.
B)Very strong nucleophiles often yield the kinetic product.
C)The reaction pathway with the lowest activation energy leads to the thermodynamic product.
D)Irreversible addition of a nucleophile favors the product that is formed most rapidly.
E)The conjugate addition product is more stable.
A)Reversible addition of a nucleophile generally favors conjugate addition.
B)Very strong nucleophiles often yield the kinetic product.
C)The reaction pathway with the lowest activation energy leads to the thermodynamic product.
D)Irreversible addition of a nucleophile favors the product that is formed most rapidly.
E)The conjugate addition product is more stable.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a feasible Grignard reagent?
A)
B)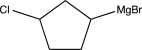
C)
D)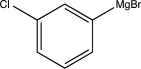
E)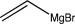
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following functional groups contains a polar π bond with no leaving group?
A)Nitrile
B)Carboxylic acid
C)Ester
D)Acid chloride
E)Amide
A)Nitrile
B)Carboxylic acid
C)Ester
D)Acid chloride
E)Amide

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following alkyl halides could not be used to generate a Wittig reagent?
A)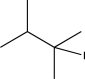
B)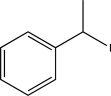
C)
D)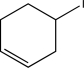
E)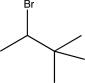
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds would you expect to react with a nucleophile at the highest rate? 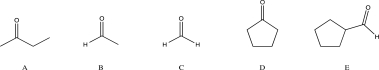
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Which nucleophile would you expect to add reversibly to an α,β-unsaturated carbonyl?
A)NaBH4
B)CH3Li
C)CH3(CH2)3MgBr
D)NaCN
E)LiAlH4
A)NaBH4
B)CH3Li
C)CH3(CH2)3MgBr
D)NaCN
E)LiAlH4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules would be unlikely to undergo a nucleophilic addition reaction? 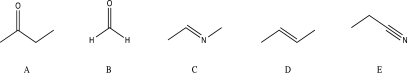
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds would you expect to have the highest percent hydration at equilibrium? 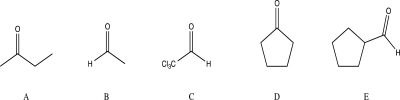
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following reactions would you expect to result in formation of the highest percentage of the conjugate addition product?
A)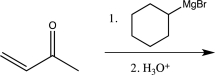
B)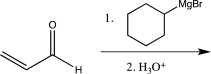
C)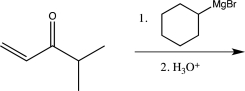
D)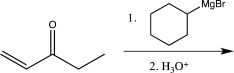
E)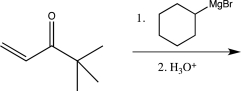
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following would not yield the alcohol shown below? 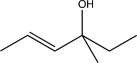
A)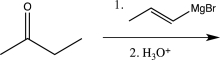
B)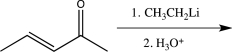
C)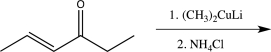
D)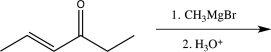
E)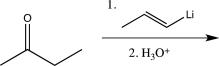

A)
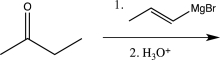
B)

C)
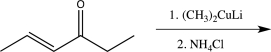
D)
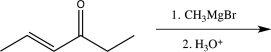
E)
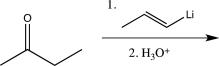

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
When the following compound is treated with dimethyl sulfide followed by sodium hydride,a product is formed whose formula is C7H12O.Draw a complete,detailed mechanism for this reaction,and predict the structure of the product. 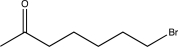


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the correct order of reactivity for this set of nucleophiles?
A)(CH3CH2)2CuLi > CH3CH2MgBr > CH3CH2Li
B)CH3CH2Li = CH3CH2MgBr > (CH3CH2)2CuLi
C)(CH3CH2)2CuLi > CH3CH2Li > CH3CH2MgBr
D)CH3CH2Li > CH3CH2MgBr > (CH3CH2)2CuLi
E)CH3CH2MgBr > (CH3CH2)2CuLi > CH3CH2Li
A)(CH3CH2)2CuLi > CH3CH2MgBr > CH3CH2Li
B)CH3CH2Li = CH3CH2MgBr > (CH3CH2)2CuLi
C)(CH3CH2)2CuLi > CH3CH2Li > CH3CH2MgBr
D)CH3CH2Li > CH3CH2MgBr > (CH3CH2)2CuLi
E)CH3CH2MgBr > (CH3CH2)2CuLi > CH3CH2Li

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Determine which of the following molecules is most susceptible to nucleophilic attack,and explain why. 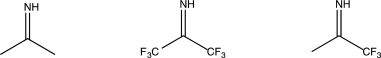


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Two students decide to use different polar aprotic solvents to carry out the reaction below.Student A chooses acetonitrile,while student B chooses tetrahydrofuran.Which is the better choice,and why? 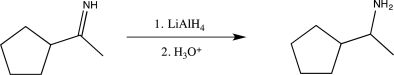


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the product of the reaction below. 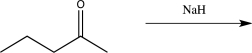


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
From the two routes shown below,choose the one that would be best for carrying out the reduction.Explain your choice. 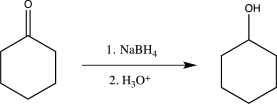
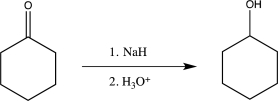



Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following reagents would be the best choice to carry out the reaction shown below? 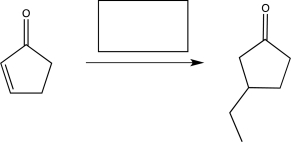
A)NaH
B)(CH3CH2)2CuLi
C)CH3MgCl
D)CH3CH2Li
E)CH3CH2MgBr

A)NaH
B)(CH3CH2)2CuLi
C)CH3MgCl
D)CH3CH2Li
E)CH3CH2MgBr

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Reductions using LiAlH4 require a separate acid workup step,while reductions using NaBH4 do not.Explain why.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following proposed syntheses could be used to produce the molecule shown below as the major product? 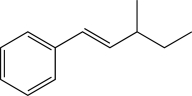
A)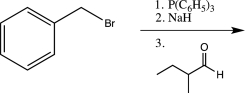
B)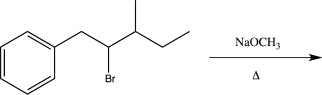
C)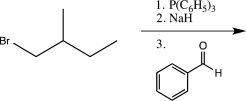
D)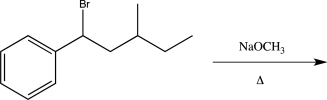
E)All of the above would be successful.

A)

B)

C)

D)

E)All of the above would be successful.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Fill in the boxes below with the appropriate reagent or product. 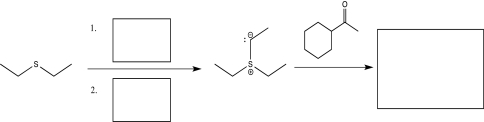


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Could the alkyl halide below be used to generate a sulfonium ylide? Show a mechanism to support your answer. 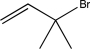


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Propose a detailed mechanism for the Knoevenagel condensation,shown below. 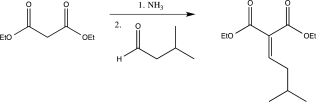


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Devise a synthesis of the compound shown below starting with ethanol and using any other reagents necessary.Provide detailed mechanisms of all reactions used. 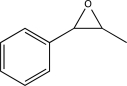


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Give one example of a hypothetical Grignard reagent that contains an incompatible functional group.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds could be synthesized via more than one possible route using a Gilman reagent?
A)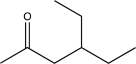
B)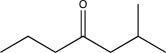
C)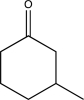
D)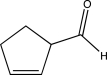
E)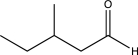
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Identify compounds X,Y,and Z in the three-step synthesis shown below. 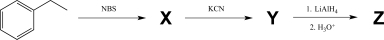


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Draw both enantiomers formed in the reaction below. 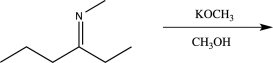


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Give an example of a ketone that would react with a nucleophile at a higher rate than acetone,and explain why it would be more reactive.
Acetone: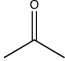
Acetone:


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
The Horner-Wadsworth-Emmons reaction is a modification of the Wittig reaction that utilizes a phosphonate-stabilized carbanion to produce an alkene.Propose a detailed mechanism for the Horner-Wadsworth-Emmons reaction shown below. 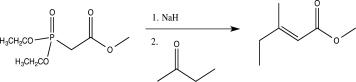


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Propose a synthesis of the following compound,starting with propionaldehyde and using any other reagents necessary.Note: This may require more than one step! 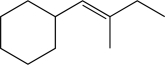


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Circle the two sites in the molecule below that are electron poor and susceptible to nucleophilic attack. 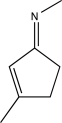


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the major organic product and provide a detailed mechanism for the reaction that would occur in the molecule shown below. 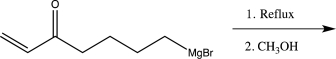


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Propose two syntheses of the following compound,each using a different Gilman reagent. 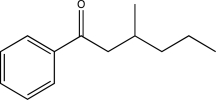


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
A student proposes the following synthetic step.Briefly explain the problem with this proposed reaction,and suggest an alternative way to synthesize the target molecule using 1-bromopropane and any other necessary reagents. 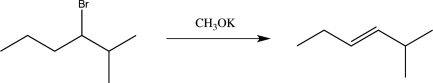


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Which is more thermodynamically stable,the product of a conjugate addition or that of a direct addition? Give two specific reasons to support your answer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Fill in the starting material needed to carry out the reaction below. 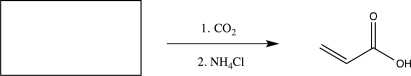


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Propose a synthesis to carry out the following transformation,using any reagents necessary.Note: This may require more than one step! 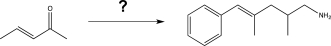


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
A reaction was carried out,and the two products shown below were obtained.Fill in the boxes with the starting material and the reagents that were used,and then draw a mechanism for the formation of the major product. 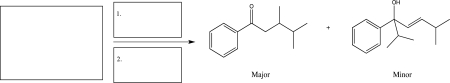


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Predict the major organic product for the following sequence of reactions. 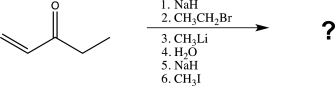
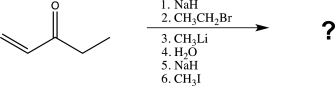

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


