Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Which of the following reactions would not result in the formation of a racemic mixture of products?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
E
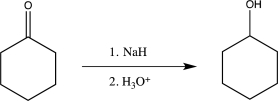
Which of the following would not yield the alcohol shown below? 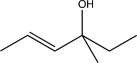
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
With respect to direct and conjugate addition,which of the following statements is false?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
C
Which nucleophile would you expect to add reversibly to an α,β-unsaturated carbonyl?
(Multiple Choice)
4.8/5  (38)
(38)
Devise a synthesis of the compound shown below starting with ethanol and using any other reagents necessary.Provide detailed mechanisms of all reactions used. 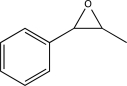
(Short Answer)
4.8/5  (47)
(47)
Give one example of a hypothetical Grignard reagent that contains an incompatible functional group.
(Essay)
4.7/5  (30)
(30)
Propose a synthesis to carry out the following transformation,using any reagents necessary.Note: This may require more than one step! 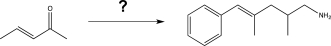
(Short Answer)
4.8/5  (32)
(32)
Circle the two sites in the molecule below that are electron poor and susceptible to nucleophilic attack. 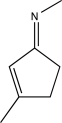
(Short Answer)
4.7/5  (31)
(31)
Fill in the starting material needed to carry out the reaction below. 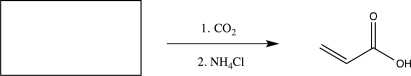
(Short Answer)
5.0/5  (44)
(44)
Which of the following compounds could be synthesized via more than one possible route using a Gilman reagent?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following proposed syntheses could be used to produce the molecule shown below as the major product? 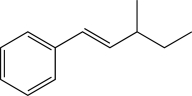
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following is the correct order of reactivity for this set of nucleophiles?
(Multiple Choice)
4.8/5  (37)
(37)
Propose two syntheses of the following compound,each using a different Gilman reagent. 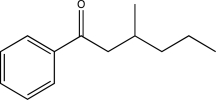
(Short Answer)
4.8/5  (33)
(33)
Which of the following solvents would be the best choice to use for the reduction of a ketone using LiAlH4?
(Multiple Choice)
4.9/5  (46)
(46)
From the two routes shown below,choose the one that would be best for carrying out the reduction.Explain your choice. 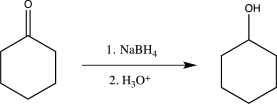
(Essay)
4.8/5  (30)
(30)
Which of the following correctly explains why LiAlH4 is a stronger reducing agent than NaBH4?
(Multiple Choice)
4.9/5  (33)
(33)
Which is more thermodynamically stable,the product of a conjugate addition or that of a direct addition? Give two specific reasons to support your answer.
(Essay)
4.8/5  (32)
(32)
Determine which of the following molecules is most susceptible to nucleophilic attack,and explain why. 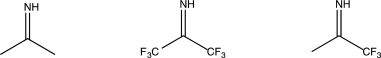
(Short Answer)
4.8/5  (38)
(38)
What is the major driving force for the mechanistic step shown below? 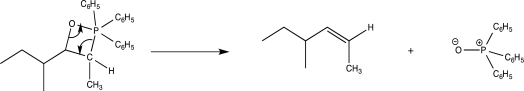
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)