Deck 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles
1
Which of the following correctly explains how sulfuric acid catalyzes the reaction shown below? 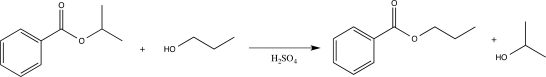
A)The acid increases the rate of nucleophile elimination.
B)The acid increases the nucleophilicity of the alcohol.
C)The acid donates a proton to the alcohol.
D)The acid converts the ester into a carboxylic acid.
E)The acid activates the carbonyl carbon and makes it more electrophilic.

A)The acid increases the rate of nucleophile elimination.
B)The acid increases the nucleophilicity of the alcohol.
C)The acid donates a proton to the alcohol.
D)The acid converts the ester into a carboxylic acid.
E)The acid activates the carbonyl carbon and makes it more electrophilic.
The acid activates the carbonyl carbon and makes it more electrophilic.
2
What is the role of PCl3 in the following reaction? 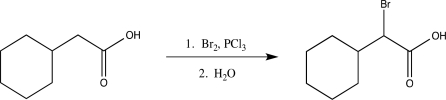
A)It converts the carboxylic acid to an enolizable acid chloride.
B)It acts as a base and deprotonates the α carbon.
C)It converts the carboxylic acid to an acid bromide.
D)It activates Br2 and forms a better nucleophile.
E)It increases the rate of the reaction.

A)It converts the carboxylic acid to an enolizable acid chloride.
B)It acts as a base and deprotonates the α carbon.
C)It converts the carboxylic acid to an acid bromide.
D)It activates Br2 and forms a better nucleophile.
E)It increases the rate of the reaction.
It converts the carboxylic acid to an enolizable acid chloride.
3
Which of the following correctly explains why two equivalents of amine are required for the aminolysis reaction shown below,while alcoholysis requires only one equivalent of alcohol? 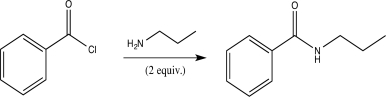
A)Amines are less nucleophilic than alcohols.
B)One equivalent of amine acts as a base and is irreversibly protonated.
C)Alcohols are stronger bases than amines.
D)Inductive effects from the Cl make the carbonyl C less electrophilic in aminolysis than in alcoholysis.
E)Two molecules of amine are involved in the rate-determining step.

A)Amines are less nucleophilic than alcohols.
B)One equivalent of amine acts as a base and is irreversibly protonated.
C)Alcohols are stronger bases than amines.
D)Inductive effects from the Cl make the carbonyl C less electrophilic in aminolysis than in alcoholysis.
E)Two molecules of amine are involved in the rate-determining step.
One equivalent of amine acts as a base and is irreversibly protonated.
4
The reaction below is an example of a(n)_________ reaction,which forms a(n)_________ as a product. 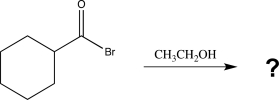
A)hydrolysis, ester
B)aminolysis, amide
C)alcoholysis, ester
D)hydrolysis, carboxylic acid
E)sulfonation, ester

A)hydrolysis, ester
B)aminolysis, amide
C)alcoholysis, ester
D)hydrolysis, carboxylic acid
E)sulfonation, ester

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds would undergo hydrolysis at the highest rate?
A)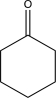
B)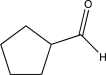
C)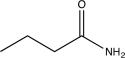
D)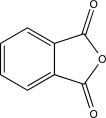
E)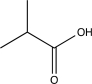
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
What is the product of the following reaction sequence? 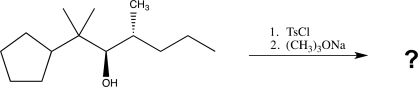
A)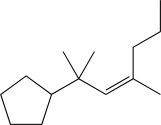
B)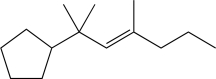
C)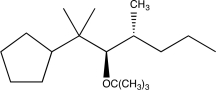
D)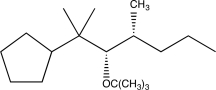
E)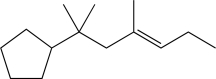

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
What is the major driving force for the reaction below? 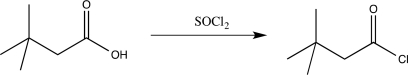
A)The product, an acid chloride, is more thermodynamically stable than a carboxylic acid.
B)Chloride is a better nucleophile than hydroxide.
C)The reaction is highly exothermic.
D)Hydroxide is a better leaving group than chloride.
E)The byproducts SO2 and HCl bubble out of solution irreversibly.

A)The product, an acid chloride, is more thermodynamically stable than a carboxylic acid.
B)Chloride is a better nucleophile than hydroxide.
C)The reaction is highly exothermic.
D)Hydroxide is a better leaving group than chloride.
E)The byproducts SO2 and HCl bubble out of solution irreversibly.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following elementary steps is involved in the mechanism of the reaction shown below? 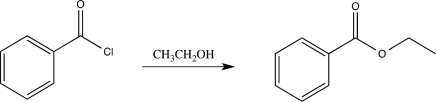
A)Coordination
B)Proton transfer
C)Bimolecular elimination
D)Electrophilic addition
E)Carbocation rearrangement

A)Coordination
B)Proton transfer
C)Bimolecular elimination
D)Electrophilic addition
E)Carbocation rearrangement

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is an intermediate in the reaction shown below? 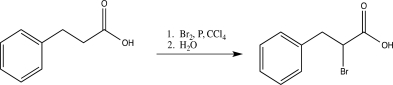
A)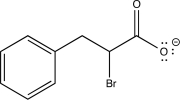
B)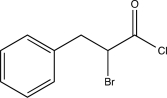
C)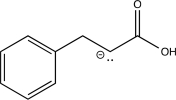
D)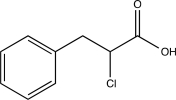
E)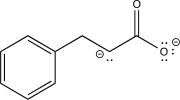

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
How many proton transfer steps occur in the mechanism for the reaction below? 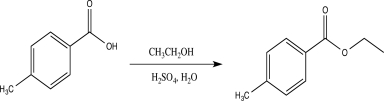
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
What base would be the best choice to catalyze the reaction shown below? 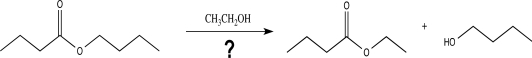
A)N(C2H5)3
B)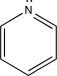
C)NaOH
D)NaO(CH2)3CH3
E)NaOCH2CH3

A)N(C2H5)3
B)

C)NaOH
D)NaO(CH2)3CH3
E)NaOCH2CH3

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
What is the rate-determining step in the reaction below? 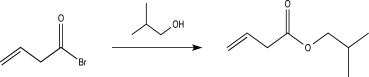
A)Proton transfer
B)Electrophilic addition
C)Nucleophilic elimination
D)Bimolecular nucleophilic substitution
E)Nucleophilic addition

A)Proton transfer
B)Electrophilic addition
C)Nucleophilic elimination
D)Bimolecular nucleophilic substitution
E)Nucleophilic addition

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
What is the starting material of the following reaction sequence? 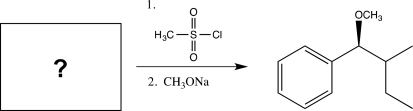
A)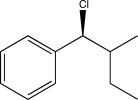
B)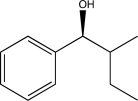
C)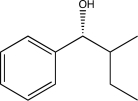
D)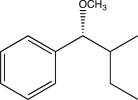
E)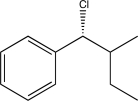

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
Determine the product of the following sequence of reactions. 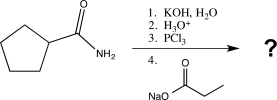
A)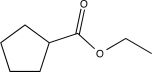
B)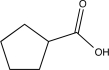
C)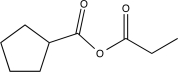
D)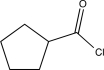
E)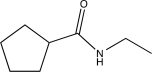

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
What is the missing reagent needed to complete the synthesis below. 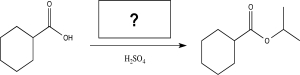
A)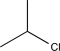
B)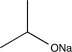
C)H2O
D)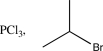
E)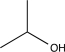

A)

B)

C)H2O
D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
What is the rate-determining step in the mechanism for the following reaction? 
A)Bimolecular nucleophilic substitution
B)Coordination
C)Nucleophile elimination
D)Nucleophilic addition
E)Proton transfer

A)Bimolecular nucleophilic substitution
B)Coordination
C)Nucleophile elimination
D)Nucleophilic addition
E)Proton transfer

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Determine the missing starting material for the sequence of reactions shown below. 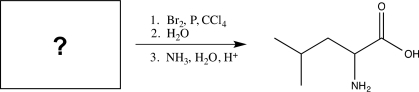
A)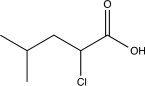
B)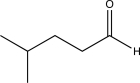
C)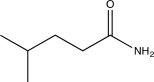
D)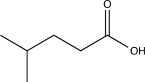
E)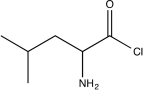

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction below is an example of a(n)________ reaction. 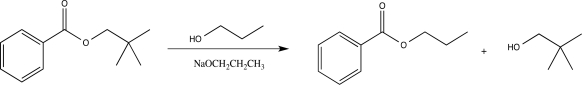
A)alcoholysis
B)base-catalyzed transesterification
C)saponification
D)acid-catalyzed transesterification
E)ester hydrolysis

A)alcoholysis
B)base-catalyzed transesterification
C)saponification
D)acid-catalyzed transesterification
E)ester hydrolysis

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the following in order of increasing reaction rate of hydrolysis. 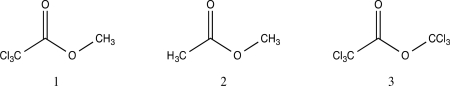
A)2 < 1 < 3
B)1 < 2 < 3
C)1 < 3 < 2
D)3 < 1 < 2
E)2 < 3 < 1

A)2 < 1 < 3
B)1 < 2 < 3
C)1 < 3 < 2
D)3 < 1 < 2
E)2 < 3 < 1

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the product of the following reaction. 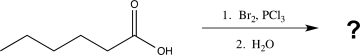
A)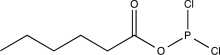
B)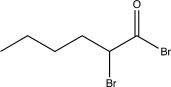
C)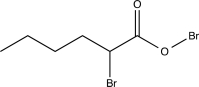
D)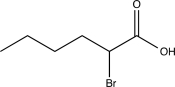
E)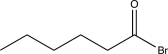

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major product of the following sequence of reactions? 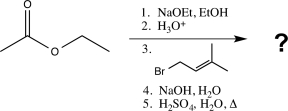
A)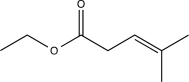
B)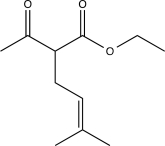
C)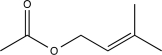
D)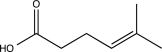
E)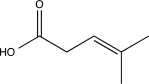

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
Propose a synthetic route to carry out the following transformation. 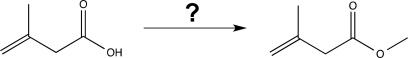
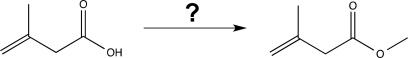

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
What is the major product of the following reaction? 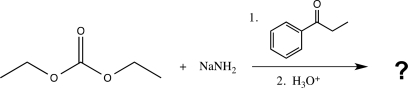
A)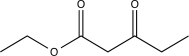
B)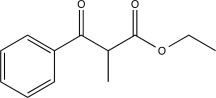
C)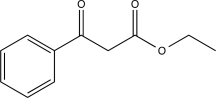
D)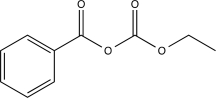
E)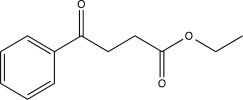
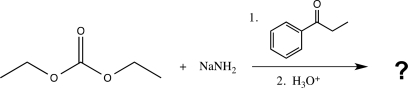
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds would produce the structure shown below when treated with a base and then acidified? 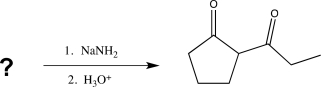
A)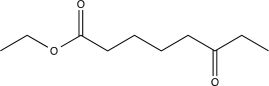
B)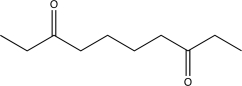
C)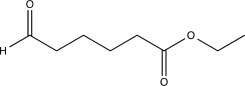
D)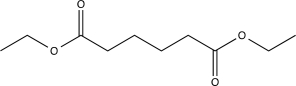
E)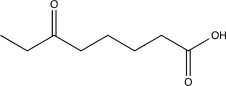

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
Draw a complete,detailed mechanism for the reaction below,and label the rate-determining step. 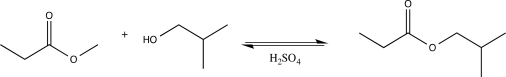


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
Determine the product(s)of the following reaction. 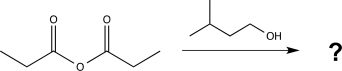


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the major product(s)of the following reaction. 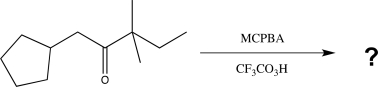
A)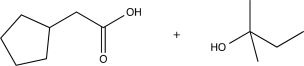
B)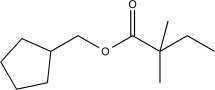
C)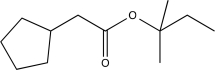
D)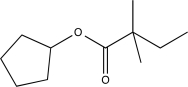
E)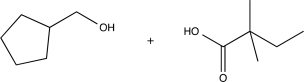

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
Show how the following could be synthesized from hexanoic acid. 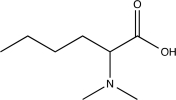


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
Indicate which of the following molecules will undergo alcoholysis at a higher rate,and explain why. 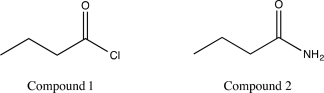


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
Draw curved arrows to complete the reaction mechanism shown below,and indicate the rate-determining step. 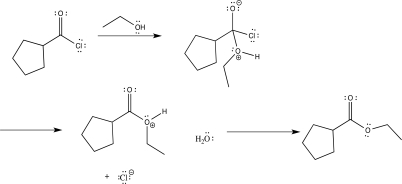


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
How many dipeptides can be formed in the reaction below? 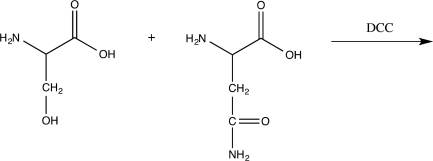
A)One
B)Two
C)Four
D)Six
E)Eight

A)One
B)Two
C)Four
D)Six
E)Eight

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
Draw a complete,detailed mechanism for the reaction below,and predict the product(s). 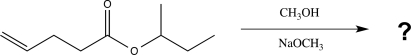


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
Draw in curved arrows and label the elementary steps in the reaction mechanism below. 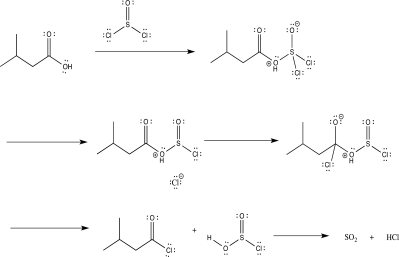


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the mechanism for the following reaction,and label each elementary step. 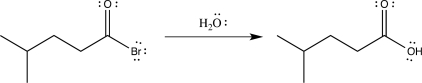


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
A student attempted to carry out the reaction below,but it was unsuccessful.Explain why the reaction did not work,and suggest a better alternative. 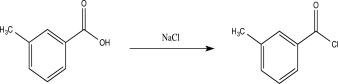


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
Show how you would carry out the following synthesis. 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
Draw a complete,detailed mechanism for the reaction below,label each elementary step,and predict the product. 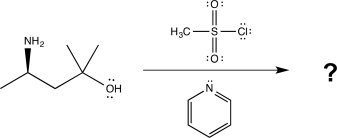


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
Draw a complete,detailed mechanism for the following reaction,and predict the products. 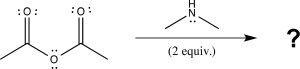


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
Briefly explain why the following reaction would be unsuccessful. 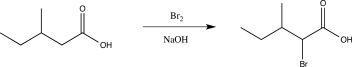


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the major product of the following reaction sequence. 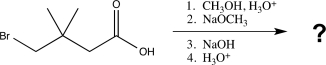
A)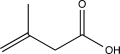
B)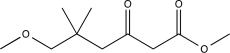
C)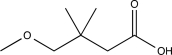
D)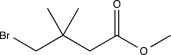
E)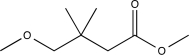
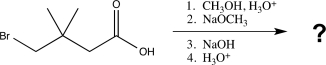
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the N-terminus,the C-terminus,and the peptide bonds in the tripeptide below. 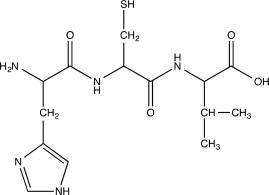


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
The acid-catalyzed transesterification reaction shown below is reversible.What are two specific things you could do to increase the yield of products? 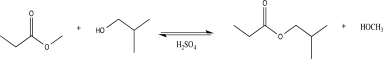


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
Fill in the missing starting material for the reaction below. 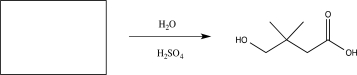


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
Suppose that a polypeptide containing 16 amino acid residues is partially hydrolyzed into three smaller peptides,A-C,which are analyzed by Edman degradation and found to have the following sequences.
A: Ala-Ile-Asn
B: Pro-Gln-Cys-Val-Pro-Val
C: Pro-Val-Asp-Ser-Gly-His-Cys-Ala-Ala
What is the original sequence of this polypeptide?
A: Ala-Ile-Asn
B: Pro-Gln-Cys-Val-Pro-Val
C: Pro-Val-Asp-Ser-Gly-His-Cys-Ala-Ala
What is the original sequence of this polypeptide?

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
Draw a complete,detailed mechanism for the following reaction,and predict the product. 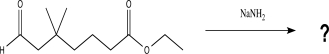


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
Draw all possible products that could be obtained from the reaction below. 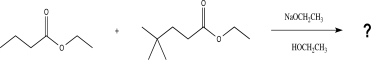


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
Fill in the boxes with an appropriate base and solvent to carry out the reaction below. 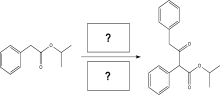


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
Draw a complete,detailed mechanism for the reaction below,and predict the product. 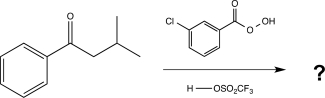


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
Fill in the missing reagents and intermediates to complete the synthesis below. 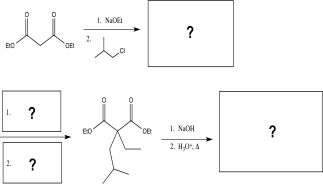


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
Show how the compound below can be synthesized using a Claisen condensation.Include all necessary reagents. 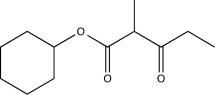


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
Predict the product of the reaction below,and identify the type of reaction. 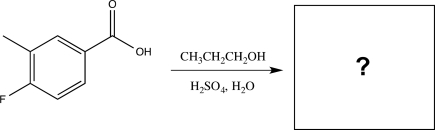


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
Show how to carry out the following synthesis using reagents with fewer than five carbon atoms. 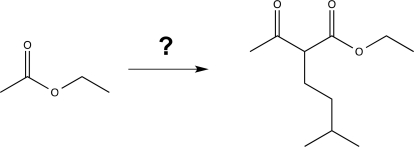


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
Show how you would carry out the following transformation,using any necessary reagents. 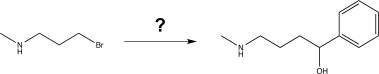


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the structures of three additional dipeptides that could be produced in the reaction below,and draw the mechanism for the production of the dipeptide shown. 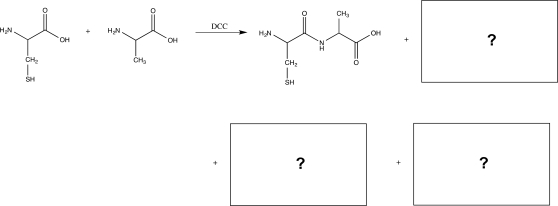


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


