Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Briefly explain why the following reaction would be unsuccessful. 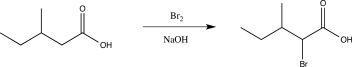
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
This reaction would be unsuccessful because the carboxylic acid proton is much more acidic than the α carbon,so it would be deprotonated by the base,and the α carbon would not be.
Draw curved arrows to complete the reaction mechanism shown below,and indicate the rate-determining step. 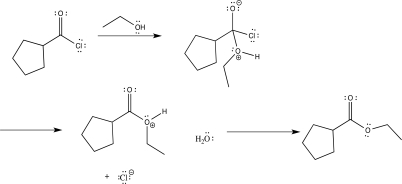
Free
(Short Answer)
4.8/5  (37)
(37)
Correct Answer:
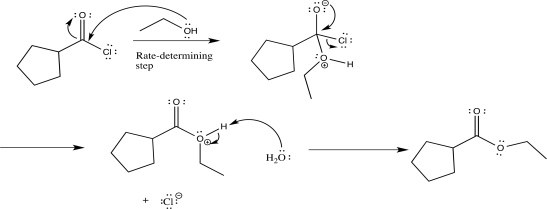
Draw a complete,detailed mechanism for the reaction below,label each elementary step,and predict the product. 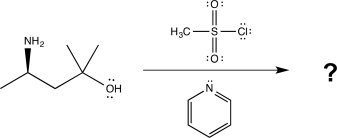
Free
(Short Answer)
4.9/5  (33)
(33)
Correct Answer:
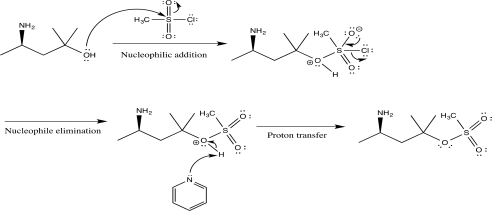
Show how you would carry out the following transformation,using any necessary reagents. 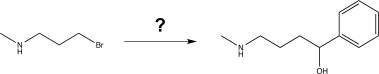
(Short Answer)
4.9/5  (34)
(34)
Draw a complete,detailed mechanism for the reaction below,and predict the product. 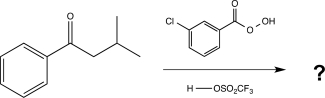
(Short Answer)
4.8/5  (42)
(42)
Draw a complete,detailed mechanism for the reaction below,and label the rate-determining step. 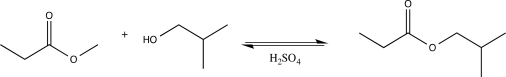
(Short Answer)
4.9/5  (40)
(40)
Suppose that a polypeptide containing 16 amino acid residues is partially hydrolyzed into three smaller peptides,A-C,which are analyzed by Edman degradation and found to have the following sequences.
A: Ala-Ile-Asn
B: Pro-Gln-Cys-Val-Pro-Val
C: Pro-Val-Asp-Ser-Gly-His-Cys-Ala-Ala
What is the original sequence of this polypeptide?
(Essay)
4.9/5  (32)
(32)
A student attempted to carry out the reaction below,but it was unsuccessful.Explain why the reaction did not work,and suggest a better alternative. 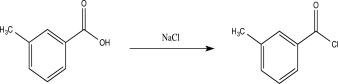
(Essay)
4.9/5  (46)
(46)
Draw a complete,detailed mechanism for the following reaction,and predict the products. 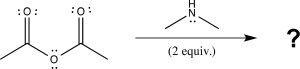
(Short Answer)
4.7/5  (44)
(44)
Which of the following correctly explains why two equivalents of amine are required for the aminolysis reaction shown below,while alcoholysis requires only one equivalent of alcohol? 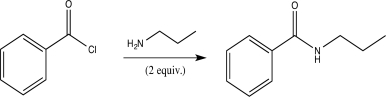
(Multiple Choice)
4.9/5  (31)
(31)
Draw a complete,detailed mechanism for the following reaction,and predict the product. 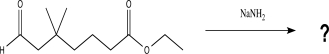
(Short Answer)
4.8/5  (36)
(36)
Predict the product of the reaction below,and identify the type of reaction. 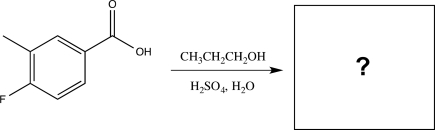
(Short Answer)
4.9/5  (38)
(38)
Draw the mechanism for the following reaction,and label each elementary step. 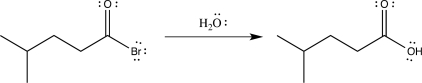
(Short Answer)
4.9/5  (40)
(40)
Which of the following compounds would produce the structure shown below when treated with a base and then acidified? 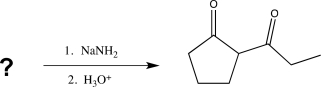
(Multiple Choice)
4.9/5  (40)
(40)
Indicate which of the following molecules will undergo alcoholysis at a higher rate,and explain why. 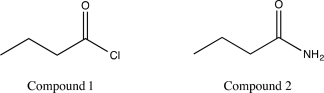
(Essay)
4.8/5  (31)
(31)
What is the major product of the following sequence of reactions? 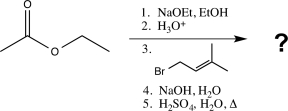
(Multiple Choice)
4.8/5  (34)
(34)
The acid-catalyzed transesterification reaction shown below is reversible.What are two specific things you could do to increase the yield of products? 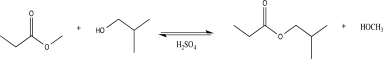
(Essay)
4.9/5  (30)
(30)
Showing 1 - 20 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)