Deck 27: Synthetic Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/116
Play
Full screen (f)
Deck 27: Synthetic Polymers
1
Which of the following addition polymers results from the reaction below? nCF2
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_6426_b657_6bcecbfae464_TB1831_11.jpg) CF2
CF2
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_6427_b657_c10f968857e3_TB1831_11.jpg) ?
?
A)[![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_8b38_b657_dd9aba5ddc0e_TB1831_11.jpg) CF-CF
CF-CF ![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_8b39_b657_59bd47563326_TB1831_11.jpg) ]n
]n
B)[CF3-CF3]n
C)[-CF2-CH![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_b24a_b657_03ada4072b50_TB1831_11.jpg) CHCF2-]n
CHCF2-]n
D)[-CF2-CF2-]n
E)[-CF2![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_b24b_b657_23be445a643b_TB1831_11.jpg) CF2-]n
CF2-]n
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_6426_b657_6bcecbfae464_TB1831_11.jpg) CF2
CF2 ![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_6427_b657_c10f968857e3_TB1831_11.jpg) ?
?A)[
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_8b38_b657_dd9aba5ddc0e_TB1831_11.jpg) CF-CF
CF-CF ![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_8b39_b657_59bd47563326_TB1831_11.jpg) ]n
]nB)[CF3-CF3]n
C)[-CF2-CH
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_b24a_b657_03ada4072b50_TB1831_11.jpg) CHCF2-]n
CHCF2-]nD)[-CF2-CF2-]n
E)[-CF2
![<strong>Which of the following addition polymers results from the reaction below? nCF<sub>2</sub> <sub> </sub> CF<sub>2 </sub> <sub> </sub> ?</strong> A)[ CF-CF ]<sub>n</sub> B)[CF<sub>3</sub>-CF<sub>3</sub>]<sub>n</sub> C)[-CF<sub>2</sub>-CH CHCF<sub>2</sub>-]<sub>n</sub> D)[-CF<sub>2</sub>-CF<sub>2</sub>-]<sub>n</sub> E)[-CF<sub>2</sub> <sub> </sub> CF<sub>2</sub>-]<sub>n</sub>](https://storage.examlex.com/TB1831/11ea8a1c_2db3_b24b_b657_23be445a643b_TB1831_11.jpg) CF2-]n
CF2-]n[-CF2-CF2-]n
2
Which of the following monomers has the greatest ability to undergo cationic polymerization? 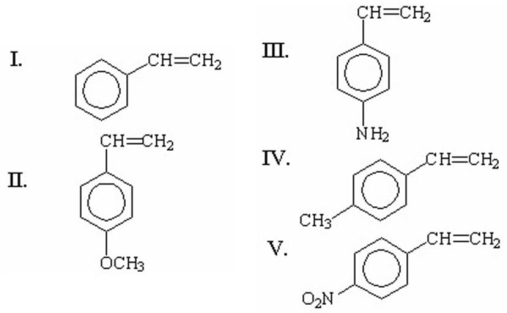
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
III
3
List the two major classes of synthetic polymers and give one example of each.
a)Addition polymers,also known as chain-growth polymers; polystyrene for example. b)Condensation polymers,also known as step-growth polymers; nylon for example.
4
Which of the following is the first chain propagating step in the radical polymerization of ethylene?
A)ROOR + CH2 CH2 → ROCH2CH2OR
CH2 → ROCH2CH2OR
B)∙ RO∙ + CH2
 CH2 → ROCH2CH2
CH2 → ROCH2CH2
C)RO∙ + CH2 CH2 → ROCH
CH2 → ROCH  CH2 + H∙
CH2 + H∙
D)∙ ∙ ROOR + CH2
 CH2 → 2 ROH + CH
CH2 → 2 ROH + CH  CH
CH
E)∙ ∙ 2RO∙ + CH2
 CH2 → 2 ROH + CH
CH2 → 2 ROH + CH  CH
CH
A)ROOR + CH2
 CH2 → ROCH2CH2OR
CH2 → ROCH2CH2ORB)∙ RO∙ + CH2
 CH2 → ROCH2CH2
CH2 → ROCH2CH2C)RO∙ + CH2
 CH2 → ROCH
CH2 → ROCH  CH2 + H∙
CH2 + H∙D)∙ ∙ ROOR + CH2
 CH2 → 2 ROH + CH
CH2 → 2 ROH + CH  CH
CHE)∙ ∙ 2RO∙ + CH2
 CH2 → 2 ROH + CH
CH2 → 2 ROH + CH  CH
CH
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the chain-initiating step in the cationic polymerization of propene? 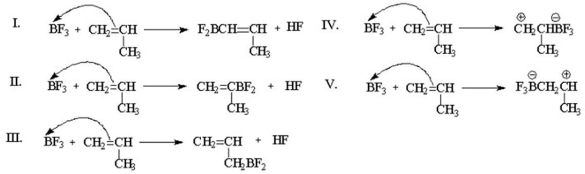
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?
A)ROOR + CH2![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_9cb4_b657_25208dcdd4f7_TB1831_11.jpg) CH2 → ROCH2CH2OR
CH2 → ROCH2CH2OR
B)CH2![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c5_b657_69865b571643_TB1831_11.jpg) CH2 + CH2
CH2 + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c6_b657_d306cf432b03_TB1831_11.jpg) CH2 → CH3CH
CH2 → CH3CH ![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c7_b657_23654ca9dcbb_TB1831_11.jpg) CHCH3
CHCH3
C)∙ RO∙ + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_ead8_b657_bb3bffe7df39_TB1831_11.jpg) CH2 → ROCH2
CH2 → ROCH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_ead9_b657_57b192fbefa0_TB1831_11.jpg) CH2
CH2
D)∙ ∙ ROCH2CH2 + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db5_11ea_b657_8f194feaf955_TB1831_11.jpg) CH2 → ROCH2CH2CH2CH2
CH2 → ROCH2CH2CH2CH2
E)∙ 2 RO-[CH2CH2]n-CH2CH2 →
RO-[CH2CH2]n-CH2CH2CH2CH2-[CH2CH2]n-OR
A)ROOR + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_9cb4_b657_25208dcdd4f7_TB1831_11.jpg) CH2 → ROCH2CH2OR
CH2 → ROCH2CH2ORB)CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c5_b657_69865b571643_TB1831_11.jpg) CH2 + CH2
CH2 + CH2![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c6_b657_d306cf432b03_TB1831_11.jpg) CH2 → CH3CH
CH2 → CH3CH ![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_c3c7_b657_23654ca9dcbb_TB1831_11.jpg) CHCH3
CHCH3C)∙ RO∙ + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_ead8_b657_bb3bffe7df39_TB1831_11.jpg) CH2 → ROCH2
CH2 → ROCH2![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db4_ead9_b657_57b192fbefa0_TB1831_11.jpg) CH2
CH2D)∙ ∙ ROCH2CH2 + CH2
![<strong>Which of the following is a possible step to terminate a chain in the radical polymerization of ethylene?</strong> A)ROOR + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>OR B)CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → CH<sub>3</sub>CH CHCH<sub>3</sub> C)∙ RO∙ + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub> <sub> </sub> CH<sub>2</sub> D)∙ ∙ ROCH<sub>2</sub>CH<sub>2</sub> + CH<sub>2</sub> <sub> </sub> CH<sub>2</sub> → ROCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub> E)∙ 2 RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub> → RO-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-[CH<sub>2</sub>CH<sub>2</sub>]<sub>n</sub>-OR](https://storage.examlex.com/TB1831/11ea8a1c_2db5_11ea_b657_8f194feaf955_TB1831_11.jpg) CH2 → ROCH2CH2CH2CH2
CH2 → ROCH2CH2CH2CH2E)∙ 2 RO-[CH2CH2]n-CH2CH2 →
RO-[CH2CH2]n-CH2CH2CH2CH2-[CH2CH2]n-OR

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
7
Chain-growth polymerization proceeds by which of the following mechanisms?
A)radical polymerization
B)cationic polymerization
C)anionic polymerization
D)A and B
E)A,B,and C
A)radical polymerization
B)cationic polymerization
C)anionic polymerization
D)A and B
E)A,B,and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following species can best serve as an initiator for anionic polymerization?
A)CH3CH2CH2CH2Li
B)BF3
C)HOOH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2OCH2CH2CH2CH3
A)CH3CH2CH2CH2Li
B)BF3
C)HOOH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2OCH2CH2CH2CH3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements best describes what alpha olefins are?
A)polysubstituted ethylenes
B)monosubstituted ethylenes
C)disubstituted ethylenes
D)ethylenes
E)isoprenes
A)polysubstituted ethylenes
B)monosubstituted ethylenes
C)disubstituted ethylenes
D)ethylenes
E)isoprenes

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following species can best serve as an initiator for cationic polymerization?
A)ROOR
B)ROH
C)ROR
D)AlCl3
E)RCOOR
A)ROOR
B)ROH
C)ROR
D)AlCl3
E)RCOOR

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
11
The choice of mechanism in chain-growth polymerization depends on which of the following?
A)structure of the monomer and initiator used
B)structure of the polymer and initiator used
C)temperature and pressure of the reaction
D)A and B
E)A,B,and C
A)structure of the monomer and initiator used
B)structure of the polymer and initiator used
C)temperature and pressure of the reaction
D)A and B
E)A,B,and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
12
What is the structure of the monomer from which the following polymer is made? 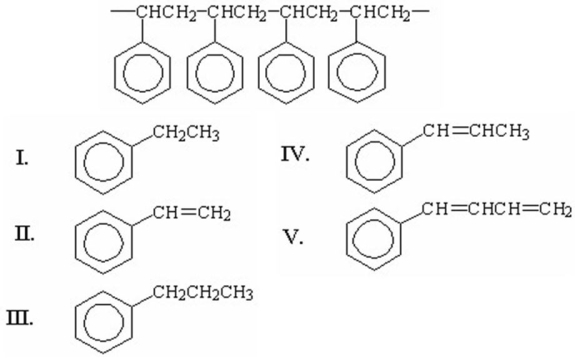
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
13
What is the structure of the monomer from which the following polymer is made? 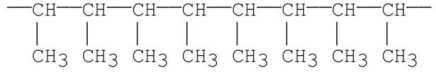
A)CH3CH3
B)CH2 CH2
CH2
C)CH3CH CH2
CH2
D)CH2 CHCH
CHCH  CH2
CH2
E)CH3CH CHCH3
CHCH3
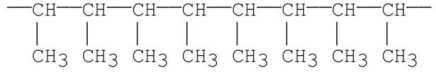
A)CH3CH3
B)CH2
 CH2
CH2C)CH3CH
 CH2
CH2D)CH2
 CHCH
CHCH  CH2
CH2E)CH3CH
 CHCH3
CHCH3
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
14
Draw the structure of the polymer and by-product that result when the two monomers below are heated together. 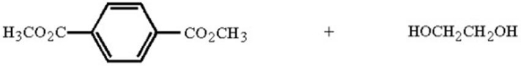
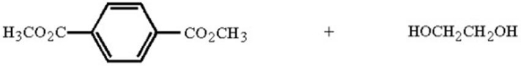

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is more likely to undergo both radical and cationic polymerization? 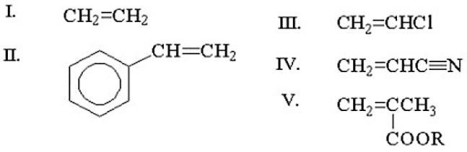
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following are characteristic of linear polyethylene?
A)They are branched polymers.
B)They are known as high-density polyethylene.
C)They are soft and flexible polymers.
D)A and B
E)A and C
A)They are branched polymers.
B)They are known as high-density polyethylene.
C)They are soft and flexible polymers.
D)A and B
E)A and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
17
Chain-growth polymerization exhibits a marked preference for
A)tail-to-tail addition
B)head-to-head addition
C)head-to-tail addition
D)head-to-middle addition
E)middle-to-tail addition
A)tail-to-tail addition
B)head-to-head addition
C)head-to-tail addition
D)head-to-middle addition
E)middle-to-tail addition

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following monomers has the greatest ability to undergo anionic polymerization? 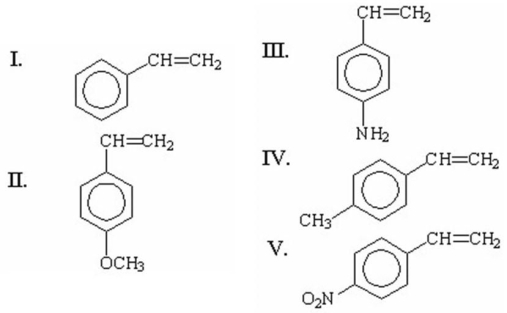
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following species can best serve as a radical initiator for radical polymerization?
A)ROH
B)ROR
C)ROOR
D)RCOOR
E)RCOOH
A)ROH
B)ROR
C)ROOR
D)RCOOR
E)RCOOH

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements best explains the process known as chain transfer?
A)a process that can control the rate of production of the polymer
B)a process that can control the molecular weight of the polymer
C)a process that can control the reactivity of the monomer
D)a process that can slow down the polymerization reaction
E)a process that can inhibit the polymerization reaction
A)a process that can control the rate of production of the polymer
B)a process that can control the molecular weight of the polymer
C)a process that can control the reactivity of the monomer
D)a process that can slow down the polymerization reaction
E)a process that can inhibit the polymerization reaction

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
21
From which of the following compounds is Lucite or poly(methyl methacrylate)prepared?
A)CH2 CHCH3
CHCH3
B)CH2 C(CH3)O2CCH3
C(CH3)O2CCH3
C)CH2 CHO2CCH3
CHO2CCH3
D)CH2 C(CH3)CO2CH3
C(CH3)CO2CH3
E)CH2 CHCO2CH3
CHCO2CH3
A)CH2
 CHCH3
CHCH3B)CH2
 C(CH3)O2CCH3
C(CH3)O2CCH3C)CH2
 CHO2CCH3
CHO2CCH3D)CH2
 C(CH3)CO2CH3
C(CH3)CO2CH3E)CH2
 CHCO2CH3
CHCO2CH3
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
22
List the three phases in the mechanism of chain-growth polymerization.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
23
Which is not typically made through radical polymerization?
A)poly(styrene)
B)PVC
C)Dacron
D)poly(vinyl acetate)
E)Lucite
A)poly(styrene)
B)PVC
C)Dacron
D)poly(vinyl acetate)
E)Lucite

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
24
In a recycling symbol,the ________ the number in the middle of the symbol,the more easily the compound can be recycled.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
25
Propose a synthesis of poly(vinyl alcohol)from H2C  CHO2CCH3.
CHO2CCH3.
 CHO2CCH3.
CHO2CCH3.
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is more likely to form non-terminated polymeric chains called living polymers?
A)cationic polymerization
B)free radical polymerization
C)anionic polymerization
D)A and B
E)A and C
A)cationic polymerization
B)free radical polymerization
C)anionic polymerization
D)A and B
E)A and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
27
When 2,2-dimethyloxirane (see figure below)undergoes anionic polymerization,the nucleophile attacks the least substituted carbon of the epoxide,but when it undergoes cationic polymerization,the nucleophile attacks the most substituted carbon of the epoxide.Explain why this is so. 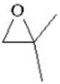


Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
28
When 3-methyl-1-butene
CH2
 CHCH(CH3)2
CHCH(CH3)2
undergoes cationic polymerization,the following expected carbocation is formed.
⊕
X-CH2-CH-CH(CH3)2
However,the following carbocation is also found in the reaction.
⊕
X-CH2-CH2-C(CH3)2
Explain.
CH2
 CHCH(CH3)2
CHCH(CH3)2undergoes cationic polymerization,the following expected carbocation is formed.
⊕
X-CH2-CH-CH(CH3)2
However,the following carbocation is also found in the reaction.
⊕
X-CH2-CH2-C(CH3)2
Explain.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
29
What structural characteristic is shared by compounds used as radical initiators?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
30
Show how branching could occur during the free-radical polymerization of styrene.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
31
What are common factors that enter into the choice of radical initiators for chain-growth polymerization?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
32
Provide a mechanism to show how H2C  CHCO2CH3 is polymerized using butyllithium as the initiator.
CHCO2CH3 is polymerized using butyllithium as the initiator.
 CHCO2CH3 is polymerized using butyllithium as the initiator.
CHCO2CH3 is polymerized using butyllithium as the initiator.
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following monomers is least likely to undergo cationic polymerization?
A)propylene
B)isobutylene
C)styrene
D)methyl acrylate
E)vinyl acetate
A)propylene
B)isobutylene
C)styrene
D)methyl acrylate
E)vinyl acetate

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
34
Explain why some monomers are better able to undergo chain-growth polymerization by a radical mechanism.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
35
Provide a mechanism to show how H2C  C(CH3)2 is polymerized using BF3 as the initiator.
C(CH3)2 is polymerized using BF3 as the initiator.
 C(CH3)2 is polymerized using BF3 as the initiator.
C(CH3)2 is polymerized using BF3 as the initiator.
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
36
Show the mechanism for the formation of a segment of polystyrene containing two molecules of styrene and initiated by hydrogen peroxide.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following can result in chain termination in cationic polymerization?
A)loss of H+
B)addition of a nucleophile that reacts with the propagating site
C)a chain transfer reaction with the solvent
D)a 1,2-hydride shift.
E)A,B,and C
A)loss of H+
B)addition of a nucleophile that reacts with the propagating site
C)a chain transfer reaction with the solvent
D)a 1,2-hydride shift.
E)A,B,and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
38
Show the three methods by which the following radical can be terminated. 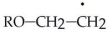
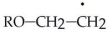

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
39
Show the reaction which occurs when benzoyl peroxide is heated.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the structure of the monomer from which Orlon [poly(acrylonitrile)] is prepared.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the monomer for poly(vinyl acetate).
A)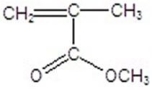
B)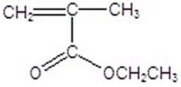
C)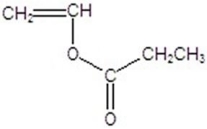
D)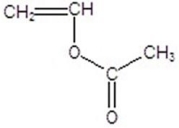
E)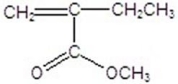
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
42
Provide the structure of poly(propylene).

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
43
What is the main structural difference between high-density and low-density polyethylene,and how does this structural difference affect the properties and uses of these polymers?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the structure of the polymer that results when methyl α-cyanoacrylate undergoes polymerization.By what name is this polymer commonly known?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the monomer(s)that undergo anionic polymerization.You may choose more than one answer.
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
46
Provide the structure of poly(vinyl chloride).

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
47
Give the monomer for the polymer teflon.
A)propylene
B)acrylonitrile
C)tetrafluoroethylene
D)poly(vinyl acetate)
E)styrene
A)propylene
B)acrylonitrile
C)tetrafluoroethylene
D)poly(vinyl acetate)
E)styrene

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the initiator in a radical polymerization.
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the monomer for superglue.
A)acrylamide
B)methyl a-cyanoacrylate
C)acrolein
D)methyl vinyl ether
E)styrene
A)acrylamide
B)methyl a-cyanoacrylate
C)acrolein
D)methyl vinyl ether
E)styrene

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
50
Draw a short segment of neoprene,using four monomers.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
51
Is acrylonitrile more likely to undergo anionic or cationic polymerization? Explain your answer.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the monomer(s)that undergo cationic polymerization.You may choose more than one answer.
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
53
What advantage does a Lewis acid like BF3 have over a proton-donating acid like HCl as a initiator in cationic polymerization?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
54
Why is chain branching less common in anionic polymerization than in cationic polymerization?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the structure of poly(acrylonitrile).

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
56
Draw a short segment of plexiglass,using five monomers.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
57
Identify the initiator in a cationic polymerization.
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the initiator in an anionic polymerization.
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl
A)KOH
B)BuLi
C)CH3OOCH3
D)BF3,H2O
E)HCl

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
59
What is the mass of each repeating unit in poly(ethylene)?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the monomer(s)that undergo radical polymerization.You may choose more than one answer.
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide
A)styrene
B)methyl methacrylate
C)vinyl chloride
D)methyl vinyl ether
E)acrylamide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
61
The pattern below is found in what type of polymer? AABBBABAABABBAAB
A)an alternating copolymer
B)a block copolymer
C)a random copolymer
D)a graft copolymer
E)a homopolymer
A)an alternating copolymer
B)a block copolymer
C)a random copolymer
D)a graft copolymer
E)a homopolymer

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
62
Draw the structure of the polymer produced in the following reaction. 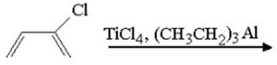
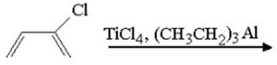

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
63
ABS is a copolymer of what three monomers?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the type of polymer for nylon 66.
A)homopolymer
B)graft copolymer
C)random copolymer
D)block copolymer
E)alternating copolymer
A)homopolymer
B)graft copolymer
C)random copolymer
D)block copolymer
E)alternating copolymer

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
65
The pattern below is found in what type of polymer? AAAABBBBAAAABBBB
A)an alternating copolymer
B)a block copolymer
C)a random copolymer
D)a graft copolymer
E)a homopolymer
A)an alternating copolymer
B)a block copolymer
C)a random copolymer
D)a graft copolymer
E)a homopolymer

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the structure of Saran,an alternating copolymer of H2C  CHCl and H2C
CHCl and H2C  CCl2.
CCl2.
 CHCl and H2C
CHCl and H2C  CCl2.
CCl2.
Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following polymers is arranged in an isotactic configuration? 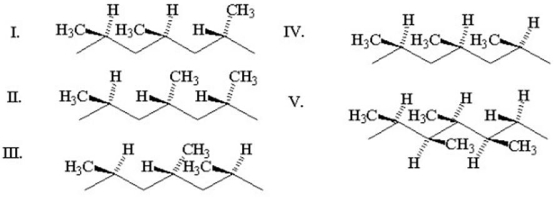
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
68
Natural rubber is a
A)polyamide.
B)polyester.
C)polycarbonate.
D)polyurethane.
E)none of the above.
A)polyamide.
B)polyester.
C)polycarbonate.
D)polyurethane.
E)none of the above.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
69
From which of the following monomers is neoprene synthesized?
A)(Z)-2-chloro-2-butene
B)(E)-2-chloro-2-butene
C)(E)-1-chloro-1,3-butadiene
D)2-chloro-1,3-butadiene
E)2,3-dichloro-1,3-butadiene
A)(Z)-2-chloro-2-butene
B)(E)-2-chloro-2-butene
C)(E)-1-chloro-1,3-butadiene
D)2-chloro-1,3-butadiene
E)2,3-dichloro-1,3-butadiene

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
70
What are aramides?
A)aromatic polyester polymers
B)aromatic amine polymers
C)alkyl halide polymers
D)aromatic polyamide polymers
E)aliphatic polyamide polymers
A)aromatic polyester polymers
B)aromatic amine polymers
C)alkyl halide polymers
D)aromatic polyamide polymers
E)aliphatic polyamide polymers

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
71
Identify the type of polymer for poly(vinyl chloride).
A)homopolymer
B)graft copolymer
C)random copolymer
D)block copolymer
E)alternating copolymer
A)homopolymer
B)graft copolymer
C)random copolymer
D)block copolymer
E)alternating copolymer

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
72
What is the difference between natural rubber and Gutta-percha rubber?
A)Natural rubber is hard and brittle while gutta-percha is soft and rubbery.
B)Natural rubber is natural while gutta-percha is synthetic rubber.
C)Natural rubber has a cis configuration while gutta-percha has a trans configuration.
D)Natural rubber and gutta-percha rubber are enantiomers.
E)The monomer for natural rubber is isoprene while the monomer for gutta-percha is 1,3-butadiene.
A)Natural rubber is hard and brittle while gutta-percha is soft and rubbery.
B)Natural rubber is natural while gutta-percha is synthetic rubber.
C)Natural rubber has a cis configuration while gutta-percha has a trans configuration.
D)Natural rubber and gutta-percha rubber are enantiomers.
E)The monomer for natural rubber is isoprene while the monomer for gutta-percha is 1,3-butadiene.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
73
Kevlar is an example of
A)a polyester.
B)an aramide.
C)a polyurethane.
D)a epoxy resin.
E)a plasticizer.
A)a polyester.
B)an aramide.
C)a polyurethane.
D)a epoxy resin.
E)a plasticizer.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following statements best describes what vulcanization means?
A)a process by which rigid and non-sticky rubber can be made soft and sticky
B)a chemical process discovered by Charles Schultz in 1944
C)the heating of rubber with sulfur
D)a chemical process that destroys cross-linking in rubber
E)all of the above
A)a process by which rigid and non-sticky rubber can be made soft and sticky
B)a chemical process discovered by Charles Schultz in 1944
C)the heating of rubber with sulfur
D)a chemical process that destroys cross-linking in rubber
E)all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
75
Provide the structure of poly(tetrafluoroethylene).

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following statements concerning the stereochemistry of polymerization is correct?
A)Polymers in the atactic configuration are more likely to be crystalline solids because regular packing of the polymer chains is easier.
B)Polymers in the isotactic configuration tend to be softer and more flexible than atactic polymers.
C)In general,radical polymerization leads primarily to branched polymers in the syndiotactic configuration.
D)The percentage of chains in the isotactic or syndiotactic configuration decreases as the polymerization temperature decreases.
E)none of the above
A)Polymers in the atactic configuration are more likely to be crystalline solids because regular packing of the polymer chains is easier.
B)Polymers in the isotactic configuration tend to be softer and more flexible than atactic polymers.
C)In general,radical polymerization leads primarily to branched polymers in the syndiotactic configuration.
D)The percentage of chains in the isotactic or syndiotactic configuration decreases as the polymerization temperature decreases.
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is a step-growth polymer?
A)polyethylene
B)polyester
C)polypropylene
D)polystyrene
E)Plexiglass
A)polyethylene
B)polyester
C)polypropylene
D)polystyrene
E)Plexiglass

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
78
A substituted ethylene polymer in which the substituents are randomly oriented is called
A)a ditactic polymer.
B)an eutatic polymer.
C)an isotactic polymer.
D)a syndiotatic polymer.
E)an atactic polymer.
A)a ditactic polymer.
B)an eutatic polymer.
C)an isotactic polymer.
D)a syndiotatic polymer.
E)an atactic polymer.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
79
Name the monomer used in the production of neoprene.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following initiators are known as Ziegler-Natta catalysts,which were discovered in 1953 in order to control the stereochemistry of polymers?
A)Al/Ti initiators
B)Ni/Cd initiators
C)Zn/Hg initiators
D)Pb/C initiators
E)Pb/PbO2 initiators
A)Al/Ti initiators
B)Ni/Cd initiators
C)Zn/Hg initiators
D)Pb/C initiators
E)Pb/PbO2 initiators

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck


