Deck 1: Keys to the Study of Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 1: Keys to the Study of Chemistry
1
Which one of the following is a "substance" in the sense of the word as used in your textbook?
A)air
B)tap water
C)sea water
D)water
E)toothpaste
A)air
B)tap water
C)sea water
D)water
E)toothpaste
water
2
Which of the following activities is not a part of good science?
A)proposing a theory
B)developing a hypothesis
C)making quantitative observations
D)designing experiments
E)indulging in speculation
A)proposing a theory
B)developing a hypothesis
C)making quantitative observations
D)designing experiments
E)indulging in speculation
indulging in speculation
3
Which of the following represents the largest volume?
A)10,000 µL
B)1000 pL
C)100 mL
D)10 nL
E)10 cm3
A)10,000 µL
B)1000 pL
C)100 mL
D)10 nL
E)10 cm3
100 mL
4
Which of the following is a chemical change?
A)boiling of water
B)melting wax
C)broiling a steak on a grill
D)condensing water vapor into rainfall
E)carving a piece of wood
A)boiling of water
B)melting wax
C)broiling a steak on a grill
D)condensing water vapor into rainfall
E)carving a piece of wood

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
The density of mercury,the only metal to exist as a liquid at room temperature,is 13.6 g/cm3.What is that density in pounds per cubic inch?
A)849 lb/in3
B)491 lb/in3
C)376 lb/in3
D)0.491 lb/in3
E)1.83 10-3 lb/in3
A)849 lb/in3
B)491 lb/in3
C)376 lb/in3
D)0.491 lb/in3
E)1.83 10-3 lb/in3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
The distance between carbon atoms in ethylene is 134 picometers.Which of the following expresses that distance in meters?
A)1.34 10-13 m
B)1.34 10-12 m
C)1.34 10-10 m
D)1.34 10-7 m
E)1.34 10-6 m
A)1.34 10-13 m
B)1.34 10-12 m
C)1.34 10-10 m
D)1.34 10-7 m
E)1.34 10-6 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
The mass of a sample is 550 milligrams.Which of the following expresses that mass in kilograms?
A)5.5 108 kg
B)5.5 105 kg
C)5.5 10-4 kg
D)5.5 10-6 kg
E)5.5 10-1 kg
A)5.5 108 kg
B)5.5 105 kg
C)5.5 10-4 kg
D)5.5 10-6 kg
E)5.5 10-1 kg

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Water vapor is less dense than ice because
A)molecules in the gas phase are in constant motion.
B)molecules in the gas phase have more potential energy than in solids.
C)molecules in the gas phase have more kinetic energy than in solids.
D)gaseous molecules have less mass.
E)molecules in the gas phase have more space between them than in solids.
A)molecules in the gas phase are in constant motion.
B)molecules in the gas phase have more potential energy than in solids.
C)molecules in the gas phase have more kinetic energy than in solids.
D)gaseous molecules have less mass.
E)molecules in the gas phase have more space between them than in solids.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
Select the best statement.
A)Chemical changes provide the only valid basis for identification of a substance.
B)Chemical changes are easily reversed by altering the temperature of the system.
C)Chemical changes always produce substances different from the starting materials.
D)Chemical changes are associated primarily with extensive properties.
E)Chemical changes are accompanied by changes in the total mass of the substances involved.
A)Chemical changes provide the only valid basis for identification of a substance.
B)Chemical changes are easily reversed by altering the temperature of the system.
C)Chemical changes always produce substances different from the starting materials.
D)Chemical changes are associated primarily with extensive properties.
E)Chemical changes are accompanied by changes in the total mass of the substances involved.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
The average distance between the Earth and the Moon is 240,000 miles.Express this distance in kilometers.
A)6.1 105 km
B)5.3 105 km
C)3.9 l05 km
D)1.5 105 km
E)9.4 104 km
A)6.1 105 km
B)5.3 105 km
C)3.9 l05 km
D)1.5 105 km
E)9.4 104 km

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
The speed needed to escape the pull of Earth's gravity is 11.3 km/s.What is this speed in mi/h?
A)65,500 mi/h
B)25,300 mi/h
C)18,200 mi/h
D)1,090 mi/h
E)5.02 10-3 mi/h
A)65,500 mi/h
B)25,300 mi/h
C)18,200 mi/h
D)1,090 mi/h
E)5.02 10-3 mi/h

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
A dose of medication was prescribed to be 35 microliters.Which of the following expresses that volume in centiliters?
A)3.5 105 cL
B)3.5 104 cL
C)3.5 cL
D)3.5 10-4 cL
E)3.5 10-3 cL
A)3.5 105 cL
B)3.5 104 cL
C)3.5 cL
D)3.5 10-4 cL
E)3.5 10-3 cL

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
You prepare 1000.mL of tea and transfer it to a 1.00 quart pitcher for storage.Which of the following statements is true?
A)The pitcher will be filled to 100% of its capacity with no tea spilled.
B)The pitcher will be filled to about 95% of its capacity.
C)The pitcher will be filled to about 50% of its capacity.
D)The pitcher will be completely filled and a small amount of tea will overflow.
E)The pitcher will be completely filled and most of the tea will overflow.
A)The pitcher will be filled to 100% of its capacity with no tea spilled.
B)The pitcher will be filled to about 95% of its capacity.
C)The pitcher will be filled to about 50% of its capacity.
D)The pitcher will be completely filled and a small amount of tea will overflow.
E)The pitcher will be completely filled and most of the tea will overflow.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
Given that 1 inch = 2.54 cm,1 cm3 is equal to
A)16.4 in3
B)6.45 in3
C)0.394 in3
D)0.155 in3
E)0.0610 in3
A)16.4 in3
B)6.45 in3
C)0.394 in3
D)0.155 in3
E)0.0610 in3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
In an average year the American chemical industry produces more than 9.5 million metric tons of sodium carbonate.Over half of this is used in the manufacture of glass while another third is used in the production of detergents and other chemicals.How many pounds of sodium carbonate are produced annually?
A)2.1 1010 lb
B)4.3 109 lb
C)1.1 107 lb
D)2.2 106 lb
E)2.1 104 lb
A)2.1 1010 lb
B)4.3 109 lb
C)1.1 107 lb
D)2.2 106 lb
E)2.1 104 lb

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
During the swing of a frictionless pendulum,what energy form(s)remain constant?
A)kinetic energy only
B)potential energy only
C)both kinetic energy and potential energy
D)kinetic plus potential energy
E)None of these forms remains constant.
A)kinetic energy only
B)potential energy only
C)both kinetic energy and potential energy
D)kinetic plus potential energy
E)None of these forms remains constant.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Select the best statement.
A)Physical changes may be reversed by changing the temperature.
B)Physical changes alter the composition of the substances involved.
C)Physical properties are not valid characteristics for identifying a substance.
D)Physical properties are mostly extensive in nature.
E)Physical changes are usually accompanied by chemical changes.
A)Physical changes may be reversed by changing the temperature.
B)Physical changes alter the composition of the substances involved.
C)Physical properties are not valid characteristics for identifying a substance.
D)Physical properties are mostly extensive in nature.
E)Physical changes are usually accompanied by chemical changes.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
The area of a 15-inch pizza is 176.7 in2.Express this area in square centimeters.
A)1140.cm2
B)448.8 cm2
C)96.8 cm2
D)69.57 cm2
E)27.39 cm2
A)1140.cm2
B)448.8 cm2
C)96.8 cm2
D)69.57 cm2
E)27.39 cm2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
A large pizza has a diameter of 15 inches.Express this diameter in centimeters.
A)38 cm
B)24 cm
C)18 cm
D)9.3 cm
E)5.9 cm
A)38 cm
B)24 cm
C)18 cm
D)9.3 cm
E)5.9 cm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
The average distance from Earth to the Sun is 150 megameters.What is that distance in meters?
A)1.5 108 m
B)1.5 106 m
C)1.5 105 m
D)1.5 103 m
E)1.5 10-6 m
A)1.5 108 m
B)1.5 106 m
C)1.5 105 m
D)1.5 103 m
E)1.5 10-6 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Express 96,342 m using 2 significant figures.
A)9.60 104 m
B)9.6 104 m
C)9.60 10-4 m
D)9.6 10-4 m
E)96,000.m
A)9.60 104 m
B)9.6 104 m
C)9.60 10-4 m
D)9.6 10-4 m
E)96,000.m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
The appropriate number of significant figures in the result of 15.234 15.208 is
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Talc is a mineral that has low conductivity for heat and electricity and that is not attacked by acid.It is used as talcum powder and face powder.A sample of talc weighs 35.97 g in air and 13.65 g in mineral oil (d = 1.75 g/cm3).What is the density of talc?
A)4.61 g/cm3
B)2.82 g/cm3
C)2.63 g/cm3
D)2.44 g/cm3
E)1.61 g/cm3
A)4.61 g/cm3
B)2.82 g/cm3
C)2.63 g/cm3
D)2.44 g/cm3
E)1.61 g/cm3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
The result of (3.8621 1.5630)- 5.98 is properly written as
A)0.06
B)0.056
C)0.0565
D)0.05646
E)0.056462
A)0.06
B)0.056
C)0.0565
D)0.05646
E)0.056462

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is an extensive property of oxygen?
A)boiling point
B)temperature
C)average kinetic energy of molecules
D)density
E)mass
A)boiling point
B)temperature
C)average kinetic energy of molecules
D)density
E)mass

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
The S.I.base unit of mass is
A)mg
B)g
C)kg
D)metric ton
E)lb
A)mg
B)g
C)kg
D)metric ton
E)lb

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Isopropyl alcohol,commonly known as rubbing alcohol,boils at 82.4°C.What is the boiling point in kelvins?
A)387.6 K
B)355.6 K
C)323.6 K
D)190.8 K
E)-190.8 K
A)387.6 K
B)355.6 K
C)323.6 K
D)190.8 K
E)-190.8 K

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Select the answer that expresses the result of this calculation with the correct number of significant figures and with correct units. 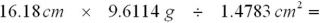
A)105.2 g/cm3
B)105.2 g/cm2
C)105.2 g/cm
D)72.13 g/cm2
E)72.13 g/cm
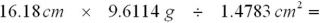
A)105.2 g/cm3
B)105.2 g/cm2
C)105.2 g/cm
D)72.13 g/cm2
E)72.13 g/cm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
The S.I.unit of speed (velocity)is
A)km/h
B)km/s
C)m/h
D)m/s
E)none of these choices is correct
A)km/h
B)km/s
C)m/h
D)m/s
E)none of these choices is correct

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is not an S.I.base unit?
A)meter
B)ampere
C)second
D)gram
E)kelvin
A)meter
B)ampere
C)second
D)gram
E)kelvin

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
The S.I.prefix mega- (M)means
A)10-6
B)10-3
C)103
D)106
E)109
A)10-6
B)10-3
C)103
D)106
E)109

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Acetone,which is used as a solvent and as a reactant in the manufacture of Plexiglas®,boils at 56.1°C.What is the boiling point in degrees Fahrenheit?
A)159°F
B)133°F
C)101°F
D)69.0°F
E)43.4°F
A)159°F
B)133°F
C)101°F
D)69.0°F
E)43.4°F

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
At a pressure of one billionth (10-9)of atmospheric pressure,there are about 2.7 1010 molecules in one cubic centimeter of a gas.How many molecules is this per cubic meter?
A)2.7 1016
B)2.7 1014
C)2.7 1012
D)2.7 108
E)2.7 104
A)2.7 1016
B)2.7 1014
C)2.7 1012
D)2.7 108
E)2.7 104

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
Which measurement is expressed to 4 significant figures?
A)0.00423 kg
B)24.049 cm
C)1300 K
D)82,306 m
E)62.40 g
A)0.00423 kg
B)24.049 cm
C)1300 K
D)82,306 m
E)62.40 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Acetic acid boils at 244.2°F.What is its boiling point in degrees Celsius?
A)382.0°C
B)167.7°C
C)153.4°C
D)117.9°C
E)103.7°C
A)382.0°C
B)167.7°C
C)153.4°C
D)117.9°C
E)103.7°C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
The appropriate number of significant figures in the result of 15.234 - 15.208 is
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
If the price of gold at the morning fixing in London was $5310 per lb,what would a kilogram of gold have cost in £ (pounds)? (Assume an exchange rate of $1.00 = £0.545)
A)£1310
B)£3510
C)£6370
D)£10400
E)£17100
A)£1310
B)£3510
C)£6370
D)£10400
E)£17100

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Select the answer that expresses the result of this calculation with the correct number of significant figures. 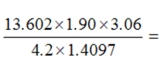
A)13.3568
B)13.357
C)13.36
D)13.4
E)13
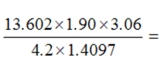
A)13.3568
B)13.357
C)13.36
D)13.4
E)13

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Select the answer with the correct number of decimal places for the following sum: 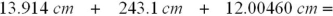
A)269.01860 cm
B)269.0186 cm
C)269.019 cm
D)269.02 cm
E)269.0 cm
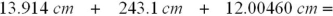
A)269.01860 cm
B)269.0186 cm
C)269.019 cm
D)269.02 cm
E)269.0 cm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
A flask has a mass of 78.23 g when empty and 593.63 g when filled with water.When the same flask is filled with concentrated sulfuric acid,H2SO4,its mass is 1026.57 g.What is the density of concentrated sulfuric acid? (Assume water has a density of 1.00 g/cm3 at the temperature of the measurement. )
A)1.992 g/cm3
B)1.840 g/cm3
C)1.729 g/cm3
D)1.598 g/cm3
E)0.543 g/cm3
A)1.992 g/cm3
B)1.840 g/cm3
C)1.729 g/cm3
D)1.598 g/cm3
E)0.543 g/cm3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
The ripening of fruit,once picked,is an example of physical change.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Write the following numbers and results in standard notation,with appropriate significant figures.
a.7.85 10-3
b.7.85 104
c.5.920 103
d.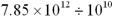
e.7.00 10-5
f.circumference of a circle,2 r,where r = 8.7 cm
g.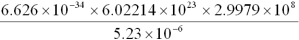
a.7.85 10-3
b.7.85 104
c.5.920 103
d.
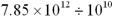
e.7.00 10-5
f.circumference of a circle,2 r,where r = 8.7 cm
g.
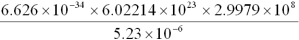

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Briefly explain the relationship between hypothesis and experiment in the scientific method.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Write the following numbers and results in scientific notation,with appropriate significant figures.
a.654
b.1234560
c.0.000000673
d.0.002590
e.200.4
f.260.0
g. r 2,where r = 8.7 cm
h.23.24 + 18.6 - 5
a.654
b.1234560
c.0.000000673
d.0.002590
e.200.4
f.260.0
g. r 2,where r = 8.7 cm
h.23.24 + 18.6 - 5

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following correctly expresses 52,030.2 m in scientific notation?
A)5.20302 104 m
B)5.20302 105 m
C)5.203 104 m
D)5.20 104 m
E)5.2 104 m
A)5.20302 104 m
B)5.20302 105 m
C)5.203 104 m
D)5.20 104 m
E)5.2 104 m

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Bud N.Chemist must determine the density of a mineral sample.His four trials yield densities of 4.77 g/cm3,4.67 g/cm3,4.69 g/cm3,and 4.81 g/cm3.Independent studies found the correct density to be 4.75 g/cm3.Which of the following statements represents the best analysis of the data?
A)Bud's results have much greater accuracy than precision.
B)Bud's results have much greater precision than accuracy.
C)Bud's results have high accuracy and high precision.
D)Bud's results have low accuracy and low precision.
E)Bud's equipment is faulty.
A)Bud's results have much greater accuracy than precision.
B)Bud's results have much greater precision than accuracy.
C)Bud's results have high accuracy and high precision.
D)Bud's results have low accuracy and low precision.
E)Bud's equipment is faulty.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
The potential energy of a car moving on a level road does not depend on its speed.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
As part of an experiment to determine the density of a new plastic developed in her laboratory,Sara Ann Dippity measures the volume of a solid sample.Her four trials yield volumes of 12.37 cm3,12.41 cm3,12.39 cm3,and 12.38 cm3.Measurements of other scientists in the lab give an average volume of 12.49 cm3.Which of the following statements represents the best analysis of the data?
A)Sara's results have low precision and high accuracy.
B)Sara's results have high precision and high accuracy.
C)Sara's results have greater precision than accuracy.
D)Sara's results have greater accuracy than precision.
E)Sara has been using a faulty instrument to measure the volume.
A)Sara's results have low precision and high accuracy.
B)Sara's results have high precision and high accuracy.
C)Sara's results have greater precision than accuracy.
D)Sara's results have greater accuracy than precision.
E)Sara has been using a faulty instrument to measure the volume.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following correctly expresses 0.000007913 g in scientific notation?
A)7.913 106 g
B)7.913 105 g
C)7.913 10-5 g
D)7.913 10-6 g
E)7.913 10-9 g
A)7.913 106 g
B)7.913 105 g
C)7.913 10-5 g
D)7.913 10-6 g
E)7.913 10-9 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
As chief chemist at Superior Analytical Products (SAP)you must design an experiment to determine the density of an unknown liquid to three (3)significant figures.The density is of the order of 1 g/cm3.You have approximately 7 mL of the liquid and only graduated cylinders and balances are available for your use.Which of the following combinations of equipment will allow you to meet but not exceed your goal?
A)Graduated cylinder with ±0.1 mL uncertainty;balance with ±0.1 g uncertainty
B)Graduated cylinder with ±0.01 mL uncertainty;balance with ±0.1 g uncertainty
C)Graduated cylinder with ±0.01 mL uncertainty;balance with ±0.01 g uncertainty
D)Graduated cylinder with ±0.001 mL uncertainty;balance with ±0.001 g uncertainty
E)Graduated cylinder with ±0.1 mL uncertainty;balance with ±0.001 g uncertainty
A)Graduated cylinder with ±0.1 mL uncertainty;balance with ±0.1 g uncertainty
B)Graduated cylinder with ±0.01 mL uncertainty;balance with ±0.1 g uncertainty
C)Graduated cylinder with ±0.01 mL uncertainty;balance with ±0.01 g uncertainty
D)Graduated cylinder with ±0.001 mL uncertainty;balance with ±0.001 g uncertainty
E)Graduated cylinder with ±0.1 mL uncertainty;balance with ±0.001 g uncertainty

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
When a wooden match burns in air,chemical potential energy is converted to kinetic energy.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Give an example of a physical property and a chemical property of each of the following:
a.oxygen gas
b.octane
c.copper
a.oxygen gas
b.octane
c.copper

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
The S.I.unit of energy is the joule,J.1 J = 1 kg·m2/s2.Another energy unit,the erg,was once in widespread use.1 erg = 1 g·cm2/s2.Calculate the number of ergs in 1 J,showing all your work.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
An important aim in much chemical work is to use macroscopic measurements in order to gain an understanding of the microscopic world.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
When applying the scientific method,it is important to avoid any form of hypothesis.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Use the relationship between temperatures in Celsius and Fahrenheit to calculate the temperature at which
a.the numerical value is the same on both scales.
b.the Fahrenheit number is exactly twice the Celsius number.
a.the numerical value is the same on both scales.
b.the Fahrenheit number is exactly twice the Celsius number.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate (to three significant figures)the numerical part of the conversion factors needed to carry out the following unit conversions:
a.density in g/cm3 to kg/m3
b.speed in mi/h to ft/s
c.area in km2 to mi2
d.area in km2 to cm2
e.mass/area of aluminum foil in mg/cm2 to g/m2
f.number of gas molecules per unit volume from /m3 to /ft3
g.number of bacteria per unit area on a microscope slide from /mm2 to /in2
a.density in g/cm3 to kg/m3
b.speed in mi/h to ft/s
c.area in km2 to mi2
d.area in km2 to cm2
e.mass/area of aluminum foil in mg/cm2 to g/m2
f.number of gas molecules per unit volume from /m3 to /ft3
g.number of bacteria per unit area on a microscope slide from /mm2 to /in2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
In each of the sets below,choose the one quantity or number which is exact.
a.(i. )the human population
(ii. )the distance in light years from the sun to Alpha Centauri,a nearby star
(iii. )the winning time for the 100 m dash in the Olympic Games
b.(i. )the weight of a particular one cent coin in g
(ii. )the boiling point of lead,in °C
(iii. )the number of cm in 1 yd
c.(i. )the measured value of the speed of light (2.998... 108 m/s)
(ii. ) (3.141... )
(iii. )the volume of milk in a 1-gallon jug
a.(i. )the human population
(ii. )the distance in light years from the sun to Alpha Centauri,a nearby star
(iii. )the winning time for the 100 m dash in the Olympic Games
b.(i. )the weight of a particular one cent coin in g
(ii. )the boiling point of lead,in °C
(iii. )the number of cm in 1 yd
c.(i. )the measured value of the speed of light (2.998... 108 m/s)
(ii. ) (3.141... )
(iii. )the volume of milk in a 1-gallon jug

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Classify the following properties of hydrogen gas as either intensive or extensive.
a.the mass of the gas sample
b.the average speed of a molecule in the sample
c.temperature
d.density
e.number of molecules present
a.the mass of the gas sample
b.the average speed of a molecule in the sample
c.temperature
d.density
e.number of molecules present

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
An evacuated 276 mL glass bulb weighs 129.6375 g.Filled with an unknown gas,the bulb weighs 130.0318 g.Calculate the gas density in g/L,and express it with an appropriate number of significant figures.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
The number 6.0448,rounded to 2 decimal places,becomes 6.05.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
The numerical value of any temperature expressed in Celsius is always different from the numerical value of the same temperature in kelvin.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
The number 6.0448,rounded to 3 decimal places,becomes 6.045.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
The weight of a coin measured as 1.96235 g on one balance is definitely more accurate than a weight measurement of 1.95 g on another balance.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
The numerical value of any temperature expressed in Celsius is always different from the numerical value of the same temperature in Fahrenheit.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
When applying the scientific method,a model or theory should be based on experimental data.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


