Exam 1: Keys to the Study of Chemistry
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The distance between carbon atoms in ethylene is 134 picometers.Which of the following expresses that distance in meters?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
The S.I.unit of energy is the joule,J.1 J = 1 kg·m2/s2.Another energy unit,the erg,was once in widespread use.1 erg = 1 g·cm2/s2.Calculate the number of ergs in 1 J,showing all your work.
Free
(Short Answer)
4.7/5  (38)
(38)
Correct Answer:
1 J = 107 erg
Acetic acid boils at 244.2°F.What is its boiling point in degrees Celsius?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
D
As chief chemist at Superior Analytical Products (SAP)you must design an experiment to determine the density of an unknown liquid to three (3)significant figures.The density is of the order of 1 g/cm3.You have approximately 7 mL of the liquid and only graduated cylinders and balances are available for your use.Which of the following combinations of equipment will allow you to meet but not exceed your goal?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following correctly expresses 52,030.2 m in scientific notation?
(Multiple Choice)
4.9/5  (38)
(38)
Bud N.Chemist must determine the density of a mineral sample.His four trials yield densities of 4.77 g/cm3,4.67 g/cm3,4.69 g/cm3,and 4.81 g/cm3.Independent studies found the correct density to be 4.75 g/cm3.Which of the following statements represents the best analysis of the data?
(Multiple Choice)
4.8/5  (35)
(35)
During the swing of a frictionless pendulum,what energy form(s)remain constant?
(Multiple Choice)
4.9/5  (40)
(40)
The numerical value of any temperature expressed in Celsius is always different from the numerical value of the same temperature in Fahrenheit.
(True/False)
4.8/5  (37)
(37)
The average distance between the Earth and the Moon is 240,000 miles.Express this distance in kilometers.
(Multiple Choice)
4.8/5  (39)
(39)
Briefly explain the relationship between hypothesis and experiment in the scientific method.
(Essay)
4.9/5  (45)
(45)
The numerical value of any temperature expressed in Celsius is always different from the numerical value of the same temperature in kelvin.
(True/False)
4.8/5  (42)
(42)
A large pizza has a diameter of 15 inches.Express this diameter in centimeters.
(Multiple Choice)
4.9/5  (40)
(40)
Calculate (to three significant figures)the numerical part of the conversion factors needed to carry out the following unit conversions:
a.density in g/cm3 to kg/m3
b.speed in mi/h to ft/s
c.area in km2 to mi2
d.area in km2 to cm2
e.mass/area of aluminum foil in mg/cm2 to g/m2
f.number of gas molecules per unit volume from /m3 to /ft3
g.number of bacteria per unit area on a microscope slide from /mm2 to /in2
(Essay)
4.8/5  (44)
(44)
Give an example of a physical property and a chemical property of each of the following:
a.oxygen gas
b.octane
c.copper
(Essay)
4.9/5  (41)
(41)
The weight of a coin measured as 1.96235 g on one balance is definitely more accurate than a weight measurement of 1.95 g on another balance.
(True/False)
4.8/5  (42)
(42)
Select the answer with the correct number of decimal places for the following sum: 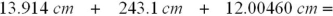
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)