Deck 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions
1
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
prop-2-enenitrile
Draw:
prop-2-enenitrile
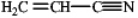
2
Instructions: Consider the data in the table below to answer the following question(s).
 Refer to instructions. Which of the acids in the table has the strongest conjugate base?
Refer to instructions. Which of the acids in the table has the strongest conjugate base?
 Refer to instructions. Which of the acids in the table has the strongest conjugate base?
Refer to instructions. Which of the acids in the table has the strongest conjugate base?CH3COOH
3
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give the major product(s):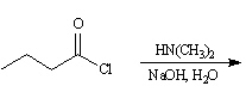
Give the major product(s):

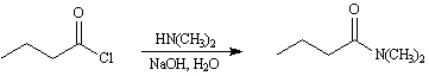
4
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):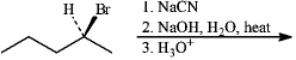
Give major product(s):
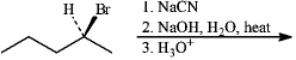

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):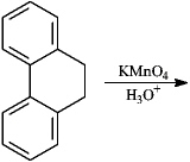
Give major product(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
2,5-dihydroxybenzoic acid
Draw:
2,5-dihydroxybenzoic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give the major product(s):
Give the major product(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give the major product(s):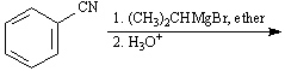
Give the major product(s):
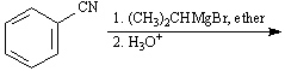

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
What is the order of increasing acidity for the following compounds? (least to most) 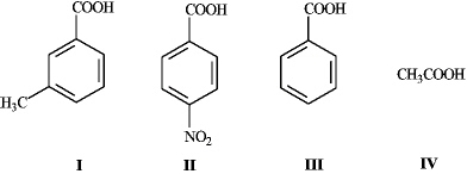
A) IV, I, III, II
B) IV, II, III, I
C) II, III, I, IV
D) I, III, II, IV

A) IV, I, III, II
B) IV, II, III, I
C) II, III, I, IV
D) I, III, II, IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
2-propylpentanoic acid
Draw:
2-propylpentanoic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: Consider the data in the table below to answer the following question(s).
 Refer to instructions. Draw the structure of the strongest acid in the table.
Refer to instructions. Draw the structure of the strongest acid in the table.
 Refer to instructions. Draw the structure of the strongest acid in the table.
Refer to instructions. Draw the structure of the strongest acid in the table.
Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
phenylacetonitrile or phenylethanenitrile
Draw:
phenylacetonitrile or phenylethanenitrile

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):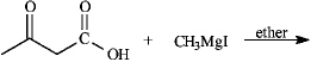
Give major product(s):
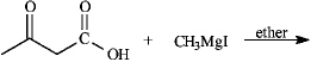

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
An ether solution contains the following components.
1,2,3,4-tetramethylbenzene benzyl alcohol 2-hydroxybenzoic acid
If this solution is extracted with a 5% solution of NaHCO3, that is immiscible with ether, which components end up in the ether layer and which in the NaHCO3 layer?
1,2,3,4-tetramethylbenzene benzyl alcohol 2-hydroxybenzoic acid
If this solution is extracted with a 5% solution of NaHCO3, that is immiscible with ether, which components end up in the ether layer and which in the NaHCO3 layer?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give the major product(s):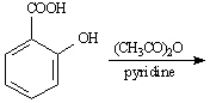
Give the major product(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Consider the data in the table below to answer the following question(s).
 Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
 Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):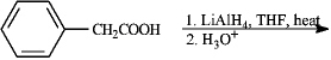
Give major product(s):
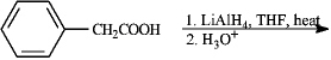

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
cis-cyclopentane-1,3-dicarboxylic acid
Draw:
cis-cyclopentane-1,3-dicarboxylic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
cyanoacetic acid
Draw:
cyanoacetic acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
N,N-dimethylformamide
Draw:
N,N-dimethylformamide

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major organic product obtained from the following reaction? 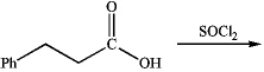
A)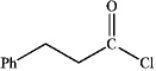
B)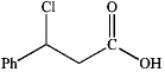
C)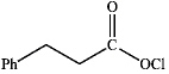
D)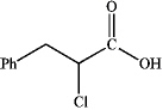

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would represent the correct reaction conditions for the following conversion? 
A) 1) KMnO4, 2) LiAlH4
B) 1) Mg, ether, 2) CO2, 3) LiAlH4
C) 1) NaOH, H2O, 2) LiAlH4
D) 1) SOCl2, benzene, 2) LiAlH4
E) either b or c

A) 1) KMnO4, 2) LiAlH4
B) 1) Mg, ether, 2) CO2, 3) LiAlH4
C) 1) NaOH, H2O, 2) LiAlH4
D) 1) SOCl2, benzene, 2) LiAlH4
E) either b or c

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Complete the following reaction sequence with the missing major organic products. 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following has the highest boiling point?
A) butanoic acid
B) butan-2-ol
C) hexanoic acid
D) heptan-3-one
A) butanoic acid
B) butan-2-ol
C) hexanoic acid
D) heptan-3-one

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Circle any of the following structures that are not considered to be derivatives of carboxylic acids. 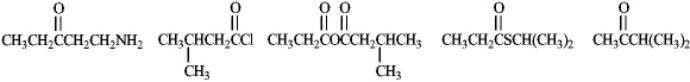
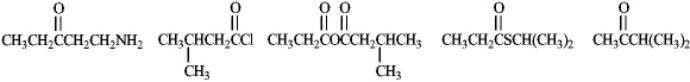

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
Instructions: Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed a Fischer esterification.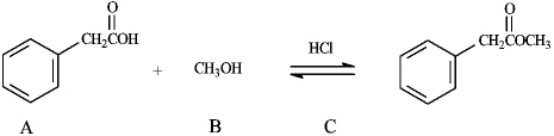 Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed a Fischer esterification.
 Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
Refer to instructions. The nucleophile in this reaction is indicated by letter _____.
Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following does not represent a similarity between nitriles and carboxylic acids?
A) contain 3 carbon bonds to an electronegative element
B) contain two p bonds.
C) act as electrophiles
D) undergo nucleophilic substitution reactions
E) all of these are characteristic of both nitriles and carboxylic acids.
A) contain 3 carbon bonds to an electronegative element
B) contain two p bonds.
C) act as electrophiles
D) undergo nucleophilic substitution reactions
E) all of these are characteristic of both nitriles and carboxylic acids.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
What is the major organic product obtained from the following reaction? 
A)
B)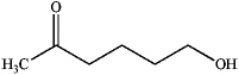
C)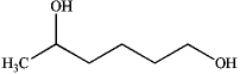
D)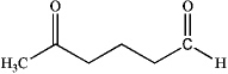

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
Rank the following compounds in order of increasing reactivity with a nucleophile. 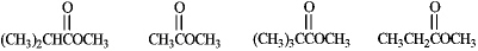
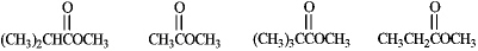

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Instructions: Provide IUPAC names for the structures in the following question(s).
Name: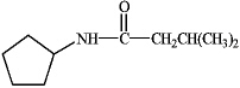
Name:


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
Instructions: Provide IUPAC names for the structures in the following question(s).
Name: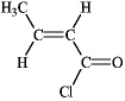
Name:


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the structure of (Z)-4-methylhex-3-enoic acid.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
Instructions: Consider the information below to answer the following question(s). The reaction of a carboxylic acid with an alcohol in the presence of acid is termed a Fischer esterification. 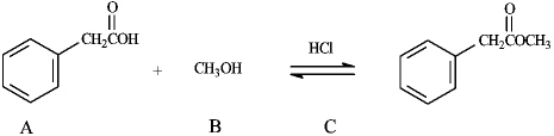 Refer to instructions. Compound C functions as _____ in this reaction.
Refer to instructions. Compound C functions as _____ in this reaction.
A) a base scavenger
B) a solvent
C) a catalyst
D) a neutralizer
 Refer to instructions. Compound C functions as _____ in this reaction.
Refer to instructions. Compound C functions as _____ in this reaction.A) a base scavenger
B) a solvent
C) a catalyst
D) a neutralizer

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
What is the correct structure for phenylbenzoate?
A)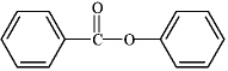
B)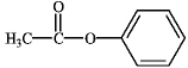
C)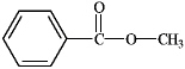
D)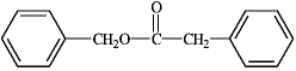
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the structure of prop-2-enamide.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the correct order of decreasing acid strength (more acidic > less acidic)?
A) FCH2COOH > CH3COOH > F2CHCOOH
B) FCH2COOH > CH3COOH > CH3OH
C) CH3CH2OH > ClCH2COOH > BrCH2COOH
D) CH3COOH > ClCH2COOH > CH3OH
A) FCH2COOH > CH3COOH > F2CHCOOH
B) FCH2COOH > CH3COOH > CH3OH
C) CH3CH2OH > ClCH2COOH > BrCH2COOH
D) CH3COOH > ClCH2COOH > CH3OH

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major organic product obtained from the following reaction? 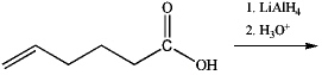
A)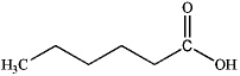
B)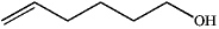
C)
D)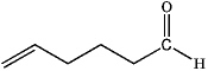

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
What is the IUPAC name of the following compound? 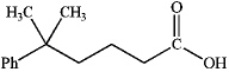
A) 5,5-dimethyl-5-phenylpentanoic acid
B) 5-methyl-5-phenylhexanoic acid
C) 2,2-dimethylphenylpropanoic acid
D) 5,5-dimethyl-5-phenylbutanal

A) 5,5-dimethyl-5-phenylpentanoic acid
B) 5-methyl-5-phenylhexanoic acid
C) 2,2-dimethylphenylpropanoic acid
D) 5,5-dimethyl-5-phenylbutanal

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 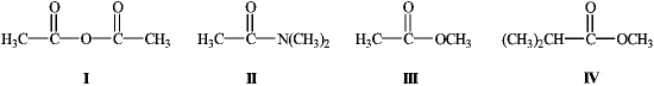
A) I, II, III, IV
B) I, III, IV, II
C) II, IV, III, I
D) II, I, III, IV
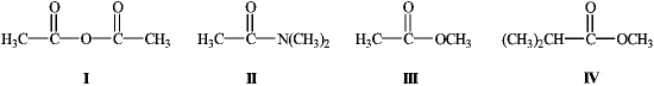
A) I, II, III, IV
B) I, III, IV, II
C) II, IV, III, I
D) II, I, III, IV

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
Instructions: Consider the information below to answer the following question(s).
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed a Fischer esterification.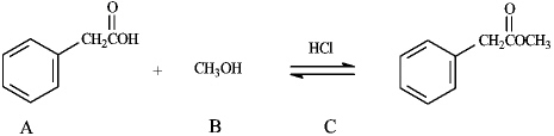 Refer to instructions. What would be the identity of A, B and C needed to produce the following compound?
Refer to instructions. What would be the identity of A, B and C needed to produce the following compound? 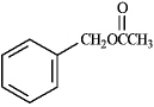
The reaction of a carboxylic acid with an alcohol in the presence of acid is termed a Fischer esterification.
 Refer to instructions. What would be the identity of A, B and C needed to produce the following compound?
Refer to instructions. What would be the identity of A, B and C needed to produce the following compound? 

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the major product of the following reaction (which affords a penicillin derivative). 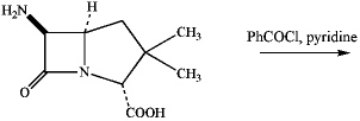


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the correct order of decreasing reactivity in hydrolysis reactions (more reactive > less reactive)?
A) anhydrides > amides > acid chlorides
B) amides > acid chlorides > anhydrides
C) anhydrides > acid chlorides > amides
D) acid chlorides > anhydrides > amides
A) anhydrides > amides > acid chlorides
B) amides > acid chlorides > anhydrides
C) anhydrides > acid chlorides > amides
D) acid chlorides > anhydrides > amides

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):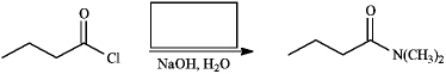
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the structure of polymer formed in the following reaction. Show only a single monomer. 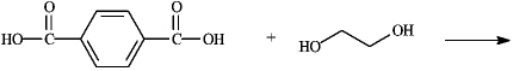
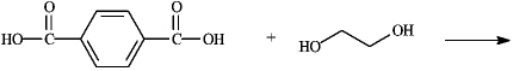

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
What is the IUPAC name of the following compound? 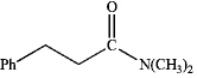
A) dimethylamino 3-phenylpropanoic acid
B) N,N-dimethyl 2-phenylethyl amide
C) N,N-dimethyl 3-phenylpropanamide
D) dimethyl 2-phenylpropanoylamine

A) dimethylamino 3-phenylpropanoic acid
B) N,N-dimethyl 2-phenylethyl amide
C) N,N-dimethyl 3-phenylpropanamide
D) dimethyl 2-phenylpropanoylamine

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the structure of polymer formed in the following reaction. Show only a single monomer. 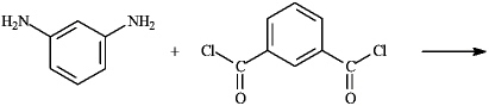


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):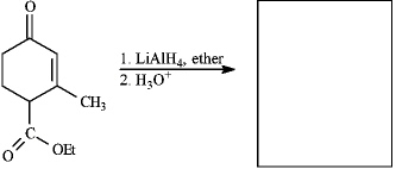
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
What is the major organic product produced by the following reaction? 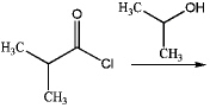
A)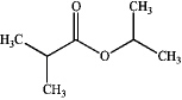
B)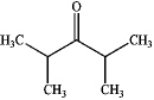
C)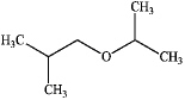
D)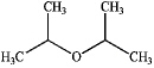

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):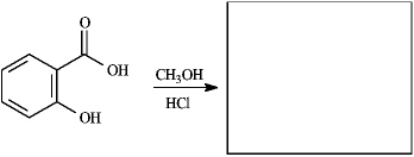
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds is a 2 amide?
A)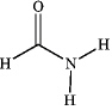
B)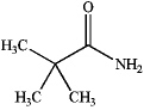
C)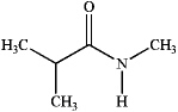
D)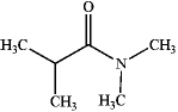
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following best describes the key mechanistic steps in the reaction of an acid chloride and an alcohol to form an ester?
A) elimination followed by addition
B) addition followed by decarboxylation
C) addition followed by elimination
D) substitution followed by addition
A) elimination followed by addition
B) addition followed by decarboxylation
C) addition followed by elimination
D) substitution followed by addition

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the major product of the following reaction. 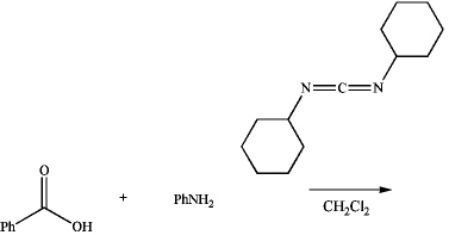


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
What is the major organic product produced by the following reaction? 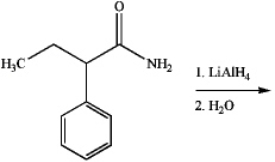


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct assignment of the functional groups in the following compounds? 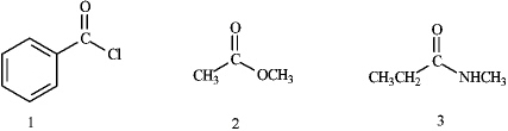
A) 1 = acid chloride; 2 = ester; 3 = nitrile
B) 1 = carboxylic acid; 2 = ester; 3 = imide
C) 1 = acid chloride; 2 = ester; 3 = amide
D) 1 = acid anhydride; 2 = ester; 3 = imide

A) 1 = acid chloride; 2 = ester; 3 = nitrile
B) 1 = carboxylic acid; 2 = ester; 3 = imide
C) 1 = acid chloride; 2 = ester; 3 = amide
D) 1 = acid anhydride; 2 = ester; 3 = imide

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the major products of the following biochemical reaction (where R = a fatty acid chain). 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):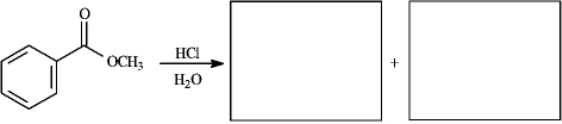
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
The product of the following reaction is a primary essence contained within bananas. 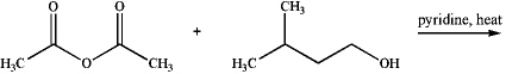

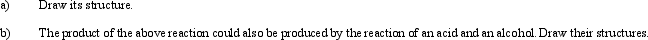

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):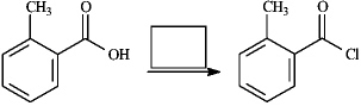
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s):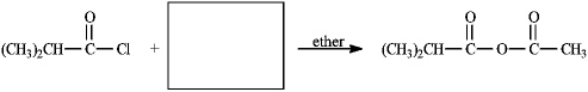
Provide missing structure(s):


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Rank the following from highest to lowest reactivity toward reaction with EtOH. 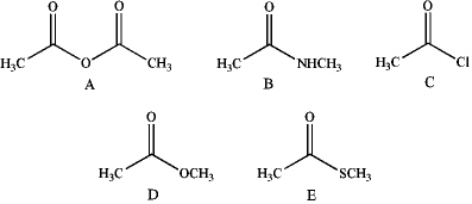


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Write the complete stepwise mechanism for the basic hydrolysis of acetamide, shown below. Show all electron flow with arrows and draw all intermediate structures. 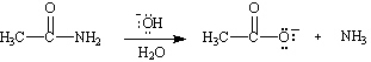


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Choose the best reagent(s) for carrying out the following conversion. 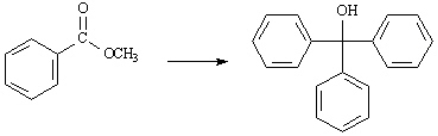




Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Choose the best reagent(s) for carrying out the following conversion. 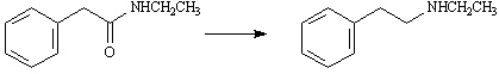




Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Choose the best reagent(s) for carrying out the following conversion. 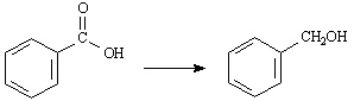




Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Ethyl phenylacetate is a pleasant smelling compound used in perfumery. Draw structures for each of the intermediates in the synthesis of ethyl phenylacetate below. 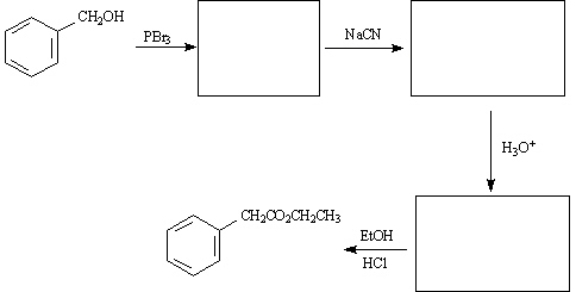


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Choose the best reagent(s) for carrying out the following conversion. 




Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


