Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Rank the following compounds in order of increasing reactivity with a nucleophile. 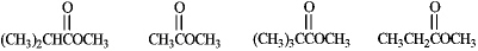
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
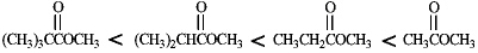
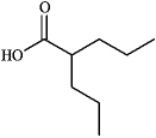
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
2-propylpentanoic acid
Free
(Essay)
4.9/5  (28)
(28)
Correct Answer:
What is the order of increasing acidity for the following compounds? (least to most) 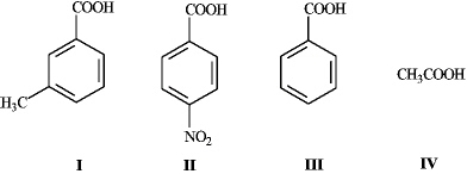
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Choose the best reagent(s) for carrying out the following conversion.  a. 1. CH3MgBr, ether
2. H O
b. CH COCl
c. CH OH, acid, heat
d. all of the above work
a. 1. CH3MgBr, ether
2. H O
b. CH COCl
c. CH OH, acid, heat
d. all of the above work
(Short Answer)
4.9/5  (40)
(40)
Which of the following best describes the key mechanistic steps in the reaction of an acid chloride and an alcohol to form an ester?
(Multiple Choice)
4.7/5  (42)
(42)
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
phenylacetonitrile or phenylethanenitrile
(Essay)
4.9/5  (38)
(38)
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s): 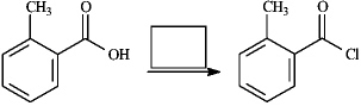
(Essay)
4.9/5  (34)
(34)
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s): 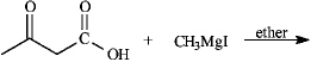
(Essay)
4.7/5  (35)
(35)
Instructions: Provide the missing structure(s) or reagent(s) for each reaction or sequences of reactions. Show all relevant stereochemistry.
Provide missing structure(s): 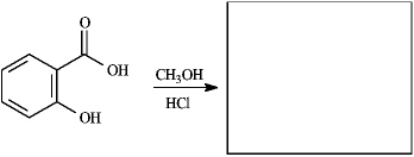
(Essay)
4.8/5  (29)
(29)
Which of the following is the correct order of decreasing acid strength (more acidic > less acidic)?
(Multiple Choice)
4.9/5  (46)
(46)
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give the major product(s): 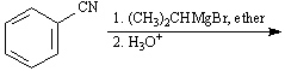
(Essay)
4.9/5  (49)
(49)
Write the complete stepwise mechanism for the basic hydrolysis of acetamide, shown below. Show all electron flow with arrows and draw all intermediate structures. 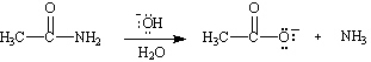
(Essay)
4.8/5  (40)
(40)
Instructions: Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s): 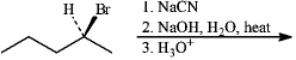
(Essay)
4.8/5  (37)
(37)
Instructions: Consider the data in the table below to answer the following question(s).
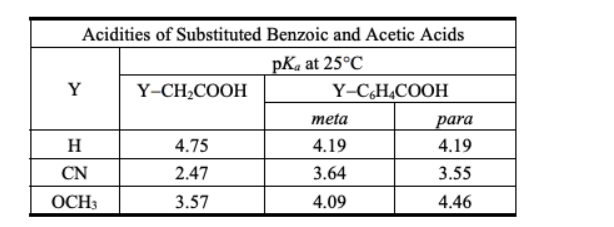 Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
(Essay)
4.8/5  (38)
(38)
Instructions: Provide IUPAC names for the structures in the following question(s).
Name: 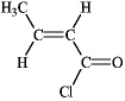
(Essay)
5.0/5  (38)
(38)
Instructions: Draw structures corresponding to each of the following IUPAC names.
Draw:
N,N-dimethylformamide
(Essay)
4.8/5  (42)
(42)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)