Deck 12: Intermolecular Forces: Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 12: Intermolecular Forces: Liquids and Solids
1
The process in which a gas is transformed into a solid is called solidification.
False
2
The phenomenon of supercooling refers to the existence of a metastable liquid at a temperature below that of its sublimation point.
False
3
H2NCH2CH2NH2 probably has a higher boiling point at 1.00 atm pressure than CH3CH2CH2NH2.
True
4
Van der Waals forces are a type of London force.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
Below are given the Lewis structures of five molecules. Which one displays the MOST hydrogen bonding?
A)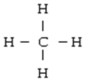
B)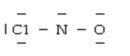
C)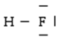
D)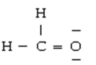
E)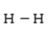
A)

B)
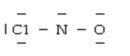
C)
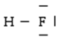
D)
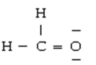
E)
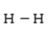

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
The heat of deposition equals the negative of the heat of sublimation.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements concerning molecules in the liquid state is true?
A) Cohesive forces are not important.
B) The molecules contract to fit the size of the container.
C) The molecules have no motion.
D) The molecules are in a patterned (oriented) arrangement.
E) The molecules are mobile and relatively close together.
A) Cohesive forces are not important.
B) The molecules contract to fit the size of the container.
C) The molecules have no motion.
D) The molecules are in a patterned (oriented) arrangement.
E) The molecules are mobile and relatively close together.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
CH4 probably has a lower boiling point at 1 atm than SnH4.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
If one compares compound A, composed of nonpolar molecules, with compound B, composed of polar molecules, and both molecules have the same molecular formula, then it is true that:
A) both compounds have the same boiling point
B) A boils at a lower temperature than B
C) B will not boil
D) B boils at a lower temperature than A
E) we have to know the structural formula to determine the differences in boiling points of A and B
A) both compounds have the same boiling point
B) A boils at a lower temperature than B
C) B will not boil
D) B boils at a lower temperature than A
E) we have to know the structural formula to determine the differences in boiling points of A and B

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
All sides are equal in length and all angles are right angles. There is an atom at each corner and one in the center. This is a simple cubic cell.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
Below are given the Lewis structures of five molecules. Which one displays the LEAST hydrogen bonding?
A)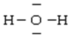
B)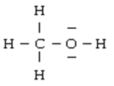
C)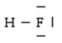
D)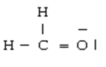
E)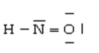
A)
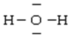
B)

C)
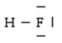
D)
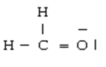
E)
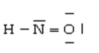

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
A liquid will "wet" a surface if:
A) the liquid has a lesser density than the surface
B) the forces between the liquid molecules are weak
C) the liquid has a low vapor pressure
D) the forces between the molecules and the surface are greater than the forces between the molecules of the liquid
E) The liquid has low viscosity and the surface is smooth
A) the liquid has a lesser density than the surface
B) the forces between the liquid molecules are weak
C) the liquid has a low vapor pressure
D) the forces between the molecules and the surface are greater than the forces between the molecules of the liquid
E) The liquid has low viscosity and the surface is smooth

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
All sides are equal in length and all angles are right angles. There is an atom at each corner and one at the center of each side. This is a face-centered cubic cell.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
Surface tension is thought to be due to:
A) surface area trying to increase
B) air absorbed on the surface
C) solute concentrated on the surface
D) surface molecules having lower energy than bulk molecules
E) surface molecules having higher energy than molecules in the bulk
A) surface area trying to increase
B) air absorbed on the surface
C) solute concentrated on the surface
D) surface molecules having lower energy than bulk molecules
E) surface molecules having higher energy than molecules in the bulk

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
Which probably has the lowest boiling point at 1.0 atm pressure?
A) PbH4
B) SnH4
C) SiH4
D) GeH4
E) CH4
A) PbH4
B) SnH4
C) SiH4
D) GeH4
E) CH4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
Which probably has the highest boiling point at 1.00 atm pressure?
A) H2NCH2CH2NH2
B) CH3CH2CH2NH2
C) (CH3)2CHNH2
D) CH3CH2NHCH3
E) (CH3)3N
A) H2NCH2CH2NH2
B) CH3CH2CH2NH2
C) (CH3)2CHNH2
D) CH3CH2NHCH3
E) (CH3)3N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
Surface tension of a liquid is the work or energy required to increase the surface area of a liquid

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds has the highest boiling point?
A) NH3
B) H2O
C) HF
D) CH4
E) HBr
A) NH3
B) H2O
C) HF
D) CH4
E) HBr

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
The property of a liquid that measures its resistance to flow is called resistivity.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
Which probably has the lowest boiling point at 1.0 atm pressure?
A) C2H6
B) C4H10
C) C6H14
D) C3H8
E) C5H12
A) C2H6
B) C4H10
C) C6H14
D) C3H8
E) C5H12

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
According to the phase diagram given, which of the following statements is wrong? 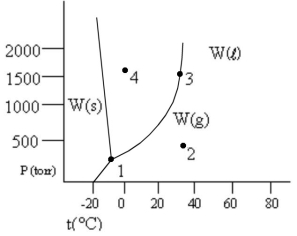
A) At the temperature and pressure of point 1, substance W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr is sufficient to liquify gaseous W.
C) If the W(l) = W(g) system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If liquid W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."

A) At the temperature and pressure of point 1, substance W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr is sufficient to liquify gaseous W.
C) If the W(l) = W(g) system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If liquid W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
According to the phase diagram given, which of the following is INCORRECT? 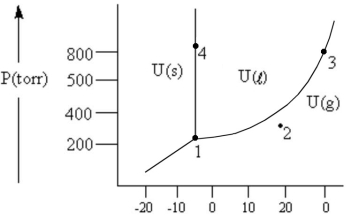
A) At the temperature and pressure of point 1, substance U exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 2, substance U exists as a one-phase gaseous system.
C) At the temperature and pressure of point 3, substance U exists as a two-phase system.
D) If the U(s)⇔ U(l) system is maintained at the temperature of point 4 while pressure is decreased steadily to about 300 torr, more U will freeze.
E) There are no conditions of temperature and pressure under which solid U will vaporize without melting first.

A) At the temperature and pressure of point 1, substance U exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 2, substance U exists as a one-phase gaseous system.
C) At the temperature and pressure of point 3, substance U exists as a two-phase system.
D) If the U(s)⇔ U(l) system is maintained at the temperature of point 4 while pressure is decreased steadily to about 300 torr, more U will freeze.
E) There are no conditions of temperature and pressure under which solid U will vaporize without melting first.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
A passage of substance directly from the vapor to the solid state is known as:
A) deposition
B) decomposition
C) sublimation
D) solidification
E) fusion
A) deposition
B) decomposition
C) sublimation
D) solidification
E) fusion

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
When a liquid is in dynamic equilibrium with its vapor at a given temperature, the following conditions could exist: I) There is no transfer of molecules between liquid and vapor.
II) The vapor pressure has a unique value.
III) The opposing processes, (liquid to vapor) and (vapor to liquid), proceed at equal rates.
IV) The concentration of vapor is dependent on time.
Which of the above choices are applicable?
A) I
B) II and III
C) I, II, and III
D) II and IV
E) II, III and IV
II) The vapor pressure has a unique value.
III) The opposing processes, (liquid to vapor) and (vapor to liquid), proceed at equal rates.
IV) The concentration of vapor is dependent on time.
Which of the above choices are applicable?
A) I
B) II and III
C) I, II, and III
D) II and IV
E) II, III and IV

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
When a liquid is in equilibrium with its vapor in a closed container:
A) the rate at which molecules from the liquid phase enter the gas phase exactly equals the rate at which molecules from the gas phase pass into the liquid phase
B) a change in temperature will not change the pressure in the container
C) the amount of gas in the container must exactly equal the amount of liquid
D) molecules cannot go from the liquid phase to the gas phase because the amount of liquid in the container is constant
E) the vapor will gradually change back to the liquid state, that is, no vapor will be left
A) the rate at which molecules from the liquid phase enter the gas phase exactly equals the rate at which molecules from the gas phase pass into the liquid phase
B) a change in temperature will not change the pressure in the container
C) the amount of gas in the container must exactly equal the amount of liquid
D) molecules cannot go from the liquid phase to the gas phase because the amount of liquid in the container is constant
E) the vapor will gradually change back to the liquid state, that is, no vapor will be left

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
The enthalpy of fusion is:
A) The quantity of heat required to melt a solid
B) The quantity of heat released when a solid melts
C) The quantity of heat when two elements are fused together to produce binary compound
D) The quantity of heat required to fuse two atomic nuclei
E) The quantity of heat released when an organic compound burns in an atmosphere of pure oxygen
A) The quantity of heat required to melt a solid
B) The quantity of heat released when a solid melts
C) The quantity of heat when two elements are fused together to produce binary compound
D) The quantity of heat required to fuse two atomic nuclei
E) The quantity of heat released when an organic compound burns in an atmosphere of pure oxygen

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
According to the phase diagram given, which of the following statements is INCORRECT? 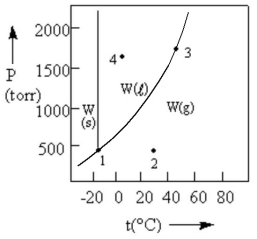
A) At the temperature and pressure of point 1, W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr will cause W to liquefy.
C) If the system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."

A) At the temperature and pressure of point 1, W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr will cause W to liquefy.
C) If the system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
Under which of the following conditions will vaporization best occur?
A) high mass, large surface area, high kinetic energy
B) weak forces between molecules, high kinetic energy, large surface area
C) high molecular energy, small surface area
D) low kinetic energy, strong molecular forces, large surface area
E) small surface area, low kinetic energy, low molecular mass
A) high mass, large surface area, high kinetic energy
B) weak forces between molecules, high kinetic energy, large surface area
C) high molecular energy, small surface area
D) low kinetic energy, strong molecular forces, large surface area
E) small surface area, low kinetic energy, low molecular mass

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements about viscosity are true: I) Viscosity is liquid's resistance to flow.
II) Viscosity decreases with a decrease in temperature.
III) Viscosity is not related to the forces between molecules in a liquid.
IV) Viscous liquids have low rate flows.
A) I) and IV)
B) I) and III)
C) I) and II)
D) II) and IV)
E) III and IV)
II) Viscosity decreases with a decrease in temperature.
III) Viscosity is not related to the forces between molecules in a liquid.
IV) Viscous liquids have low rate flows.
A) I) and IV)
B) I) and III)
C) I) and II)
D) II) and IV)
E) III and IV)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
The phenomenon in which a steel needle can, with proper care, be made to float on the surface of some water illustrates a property of liquids known as:
A) compressibility
B) polarizability
C) surface tension
D) triple point
E) viscosity
A) compressibility
B) polarizability
C) surface tension
D) triple point
E) viscosity

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
A liquid has a normal boiling point of 78°C and its vapor pressure is 400 mmHg at 50°C. To compute the molar heat of vaporization, one needs:
A) the mole weight
B) all information has been provided
C) the vapor pressure at another temperature
D) the specific heat
E) molarity
A) the mole weight
B) all information has been provided
C) the vapor pressure at another temperature
D) the specific heat
E) molarity

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following keeps the rate of vaporization unchanged?
A) closing container lid
B) increasing forces between molecules
C) increasing mass of molecule
D) decreasing temperature
E) decreasing surface area
A) closing container lid
B) increasing forces between molecules
C) increasing mass of molecule
D) decreasing temperature
E) decreasing surface area

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is true assuming point A has P = 180 mmHg, T = 50°C, point C has P = 275 mmHg, T = 200°C and points A, B, and C form a right triangle? 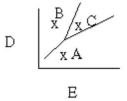
A) At point B, T = 50°C, P = 275 mmHg.
B) D is temperature, E is pressure.
C) D is pressure, E is volume.
D) At point B, T = 200°C, P = 275 mmHg.
E) B is a liquid.

A) At point B, T = 50°C, P = 275 mmHg.
B) D is temperature, E is pressure.
C) D is pressure, E is volume.
D) At point B, T = 200°C, P = 275 mmHg.
E) B is a liquid.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
What is the difference between "normal boiling point" and "boiling point" of a liquid?
A) "Normal boiling point" is a boiling point of a liquid at normal (standard) pressure and temperature, while "boiling point" is measured at any other conditions of pressure and temperature.
B) "Normal boiling point" is a boiling point of 1 mol of a liquid with a surface of 1 m2, while "boiling point" is boiling temperature for any other amount of a substance.
C) "Normal boiling point" is a boiling point a liquid has at 1 atm of pressure; "boiling point" is a boiling temperature of a liquid at any other pressure.
D) "Normal boiling point" and "boiling point" are synonyms.
E) "Normal boiling point" refers to 1 g of substance; "boiling point" refers to any other amount of substance.
A) "Normal boiling point" is a boiling point of a liquid at normal (standard) pressure and temperature, while "boiling point" is measured at any other conditions of pressure and temperature.
B) "Normal boiling point" is a boiling point of 1 mol of a liquid with a surface of 1 m2, while "boiling point" is boiling temperature for any other amount of a substance.
C) "Normal boiling point" is a boiling point a liquid has at 1 atm of pressure; "boiling point" is a boiling temperature of a liquid at any other pressure.
D) "Normal boiling point" and "boiling point" are synonyms.
E) "Normal boiling point" refers to 1 g of substance; "boiling point" refers to any other amount of substance.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
Choose the INCORRECT statement.
A) In a network covalent solid, covalent bonds extend throughout the crystalline solid.
B) Diamond has sp3 hybridization.
C) Graphite has sp hybridization.
D) Fullarenes are a recently discovered allotropic form of carbons.
E) Nanotubes are an allotropic form of carbon.
A) In a network covalent solid, covalent bonds extend throughout the crystalline solid.
B) Diamond has sp3 hybridization.
C) Graphite has sp hybridization.
D) Fullarenes are a recently discovered allotropic form of carbons.
E) Nanotubes are an allotropic form of carbon.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
A liquid is in equilibrium with its vapor. If some of the vapor is allowed to escape, what is the immediate result?
A) The condensation rate decreases.
B) The vaporization rate increases.
C) The condensation rate increases.
D) The vaporization rate decreases.
E) The rates of condensation and vaporization are not effected.
A) The condensation rate decreases.
B) The vaporization rate increases.
C) The condensation rate increases.
D) The vaporization rate decreases.
E) The rates of condensation and vaporization are not effected.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Vaporization occurs more readily with:
A) increased temperature, increased surface area, decreased volume
B) increased temperature, increased surface area, increased intramolecular forces
C) increased temperature, decreased surface area, decreased intermolecular forces
D) increased temperature, increased surface area, decreased intermolecular forces
E) increased temperature, decreased surface area, decreased intermolecular forces.
A) increased temperature, increased surface area, decreased volume
B) increased temperature, increased surface area, increased intramolecular forces
C) increased temperature, decreased surface area, decreased intermolecular forces
D) increased temperature, increased surface area, decreased intermolecular forces
E) increased temperature, decreased surface area, decreased intermolecular forces.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
According to the phase diagram given, which of the following statements is INCORRECT? 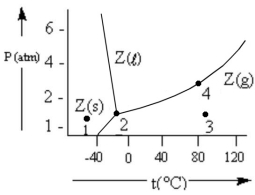
A) At the temperature and pressure of point 2, substance Z exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 3, substance Z exists as a one-phase gaseous system.
C) If the Z(s) = Z(l) = Z(g) system is maintained at the temperature of point 2 while pressure is decreased, more Z will vaporize.
D) If liquid Z is maintained at the pressure of point 4 while the temperature is decreased to 30°C, the liquid will vaporize.
E) The existence of liquid Z at -50°C and 2 atm represents the metastable condition of "supercooling."

A) At the temperature and pressure of point 2, substance Z exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 3, substance Z exists as a one-phase gaseous system.
C) If the Z(s) = Z(l) = Z(g) system is maintained at the temperature of point 2 while pressure is decreased, more Z will vaporize.
D) If liquid Z is maintained at the pressure of point 4 while the temperature is decreased to 30°C, the liquid will vaporize.
E) The existence of liquid Z at -50°C and 2 atm represents the metastable condition of "supercooling."

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
The phenomenon of supercooling refers to the existence of a metastable:
A) liquid at a temperature below that of its critical point
B) liquid at a temperature below that of its sublimation point
C) liquid at a temperature below that of its freezing point
D) gas at a temperature below that of its critical point
E) two-phase liquid/solid mixture at the freezing point
A) liquid at a temperature below that of its critical point
B) liquid at a temperature below that of its sublimation point
C) liquid at a temperature below that of its freezing point
D) gas at a temperature below that of its critical point
E) two-phase liquid/solid mixture at the freezing point

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following describe the critical point of a liquid? I) the temperature and pressure at which a liquid's meniscus disappears
II) the point where the vapor pressure curve intersects the fusion temperature curve
III) the highest temperature and pressure at which a liquid can exist
IV) the highest temperature at which it is possible to obtain a liquid from its vapor by increasing pressure
A) I), II), III)
B) I), III), IV)
C) II), III)
D) II), III), IV)
E) I), IV)
II) the point where the vapor pressure curve intersects the fusion temperature curve
III) the highest temperature and pressure at which a liquid can exist
IV) the highest temperature at which it is possible to obtain a liquid from its vapor by increasing pressure
A) I), II), III)
B) I), III), IV)
C) II), III)
D) II), III), IV)
E) I), IV)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
Three types of holes of the cubic closest packed structure are:
A) Trigonal, tetrahedral and octahedral
B) Tetrahedral, pyramidal and octahedral
C) Tetrahedral, bipyramidal and octahedral
D) Cubic,, tetrahedral, and octahedral
E) Tetragonal, tetrahedral and octahedral
A) Trigonal, tetrahedral and octahedral
B) Tetrahedral, pyramidal and octahedral
C) Tetrahedral, bipyramidal and octahedral
D) Cubic,, tetrahedral, and octahedral
E) Tetragonal, tetrahedral and octahedral

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
The property that causes water to have a concave meniscus but mercury to have a convex meniscus is ________.
A) heat of vaporization
B) sublimation
C) surface tension
D) vapor pressure
E) critical point
A) heat of vaporization
B) sublimation
C) surface tension
D) vapor pressure
E) critical point

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
The property of a liquid that measures its resistance to flow is called ________.
A) capillarity
B) polarizability
C) resistivity
D) viscosity
E) wetability
A) capillarity
B) polarizability
C) resistivity
D) viscosity
E) wetability

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
Arrange the following compounds in order of increasing boiling point: HCl, HBr, HI.
A) HCl, HBr, HI
B) HBr, HI, HCl
C) HI, HBr, HCl
D) HCl, HI, HBr
E) HI, HCl, HBr
A) HCl, HBr, HI
B) HBr, HI, HCl
C) HI, HBr, HCl
D) HCl, HI, HBr
E) HI, HCl, HBr

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
Arrange in order by decreasing boiling point: CH3CH2OH, HOCH2CH2OH, C4H10
A) HOCH2CH2OH, CH3CH2OH, C4H10
B) C4H10, HOCH2CH2OH, CH3CH2OH
C) HOCH2CH2OH, C4H10, CH3CH2OH
D) C4H10, CH3CH2OH, HOCH2CH2OH
E) CH3CH2OH, C4H10, HOCH2CH2OH
A) HOCH2CH2OH, CH3CH2OH, C4H10
B) C4H10, HOCH2CH2OH, CH3CH2OH
C) HOCH2CH2OH, C4H10, CH3CH2OH
D) C4H10, CH3CH2OH, HOCH2CH2OH
E) CH3CH2OH, C4H10, HOCH2CH2OH

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the substances below would produce the hardest, most brittle crystals?
A) SiC
B) Xe
C) Cu
D) KNO3
E) H2O
A) SiC
B) Xe
C) Cu
D) KNO3
E) H2O

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
What is the maximum number of glycerol molecules [HOCH2CH(OH)CH2OH] that could form hydrogen bonds to a single molecule of the compound?
A) 1
B) 3
C) 5
D) 9
E) 14
A) 1
B) 3
C) 5
D) 9
E) 14

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
A compound of iron and sulfur crystallizes in a lattice pattern described as cubic closest packed sulfide ions, with iron ions in all octahedral sites. What is its empirical formula?
A) FeS
B) FeS2
C) Fe3S4
D) Fe2S
E) Fe2S3
A) FeS
B) FeS2
C) Fe3S4
D) Fe2S
E) Fe2S3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
An atom at the corner of cubic unit cell is shared among:
A) six adjoining unit cells
B) four adjoining unit cells
C) eight adjoining unit cells
D) ten adjoining unit cells
E) twelve adjoining unit cells
A) six adjoining unit cells
B) four adjoining unit cells
C) eight adjoining unit cells
D) ten adjoining unit cells
E) twelve adjoining unit cells

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds is the most viscous? CH3CH2OH, CH3CH2CH2CH3, HOCH2CH2OH, CH3OCH2CH3
A) CH3CH2OH
B) CH3CH2CH2CH3
C) CH3OCH2CH3
D) HOCH2CH2OH
A) CH3CH2OH
B) CH3CH2CH2CH3
C) CH3OCH2CH3
D) HOCH2CH2OH

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
The enthalpy of vaporization at 298 K for diethylether (C4H10O) is 26.0 kJ/mol. How much heat would be required to vaporize 1.00 L of the ether at 298 K if its density is 0.714 g/L?
A) 440 J
B) 250 J
C) 186 J
D) 130 J
E) 74.1 J
A) 440 J
B) 250 J
C) 186 J
D) 130 J
E) 74.1 J

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following ionic compounds should have the highest melting point?
A) NaI
B) MgO
C) NaCl
D) LiBr
E) CaS
A) NaI
B) MgO
C) NaCl
D) LiBr
E) CaS

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
Arrange the following compounds in order of increasing boiling point: pentane (CH3CH2CH2CH2CH3), methyl butane (CH3CH(CH3)CH2CH3), neopentane (CH3C(CH3)3).
A) pentane, methyl butane, neopentane
B) neopentane, methyl butane, pentane
C) neopentane, pentane, methyl butane
D) pentane, neopentane, methyl butane
E) methyl butane, pentane, neopentane
A) pentane, methyl butane, neopentane
B) neopentane, methyl butane, pentane
C) neopentane, pentane, methyl butane
D) pentane, neopentane, methyl butane
E) methyl butane, pentane, neopentane

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
Arrange in order by decreasing boiling point: Cl2, I2, HI
A) I2, Cl2, HI
B) Cl2, I2, HI
C) Cl2, HI, I2
D) HI, Cl2, I2
E) HI, I2, Cl2
A) I2, Cl2, HI
B) Cl2, I2, HI
C) Cl2, HI, I2
D) HI, Cl2, I2
E) HI, I2, Cl2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
There are no types of crystalline solids that are held together by:
A) dipole-dipole interactions
B) ionic attractions
C) hydrogen bonds
D) covalent bonds
E) pi bonds only
A) dipole-dipole interactions
B) ionic attractions
C) hydrogen bonds
D) covalent bonds
E) pi bonds only

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
Coordination number is:
A) The number of atoms per unit cell
B) The number of atoms in the corners of the unit cell
C) The number of atoms in contact with a given atom
D) The number of atoms in the holes of closest packed structures
E) The number of layers in close packed structures
A) The number of atoms per unit cell
B) The number of atoms in the corners of the unit cell
C) The number of atoms in contact with a given atom
D) The number of atoms in the holes of closest packed structures
E) The number of layers in close packed structures

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
A crystal and its melt readily conduct electricity. The crystal also has a luster and is easily deformed. Thus, it is:
A) a covalent network crystal
B) an ionic crystal
C) a metallic crystal
D) a molecular crystal
E) a covalent crystal.
A) a covalent network crystal
B) an ionic crystal
C) a metallic crystal
D) a molecular crystal
E) a covalent crystal.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
Which combination of "type of solid" and specific example is INCORRECT?
A) ionic/"table salt"
B) metallic/copper wire
C) molecular/"dry ice"
D) network covalent/iodine
E) network covalent/silicon carbide
A) ionic/"table salt"
B) metallic/copper wire
C) molecular/"dry ice"
D) network covalent/iodine
E) network covalent/silicon carbide

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
Find a FALSE statement about X-rays.
A) diffracted by crystals
B) radiation of wavelength approximating 1 Angstrom
C) visible to the naked eye
D) used to determine the structure of molecules
E) produced using high-energy electrons
A) diffracted by crystals
B) radiation of wavelength approximating 1 Angstrom
C) visible to the naked eye
D) used to determine the structure of molecules
E) produced using high-energy electrons

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
The enthalpy of condensation is equal to, but opposite in sign from, the enthalpy of ________.
A) crystallization
B) formation
C) fusion
D) sublimation
E) vaporization
A) crystallization
B) formation
C) fusion
D) sublimation
E) vaporization

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
A 0.90 g sample of liquid water was introduced into an evacuated 2.00 L flask, which was then sealed and heated to 37°C. What percentage, by mass, of the water remained as liquid? [Vapor pressure of water at 37°C = 48.2 torr.]
A) 10%
B) 18%
C) 82%
D) 90%
E) 0%
A) 10%
B) 18%
C) 82%
D) 90%
E) 0%

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
Flask A has a volume of 0.50 L and contains 50 g of a volatile liquid. Flask B has a volume of 1.0 L and contains 25 g of the same liquid. Both flasks are stoppered and both are at 30°C. The ratio of the vapor pressure of the liquid in Flask A to that in Flask B is:
A) 4:1
B) 2:1
C) 1:1
D) 1:2
E) 1:4
A) 4:1
B) 2:1
C) 1:1
D) 1:2
E) 1:4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
Arrange the following compounds in order of increasing boiling point: I) 1-propanol, CH3CH2CH2OH;
II) 1,2-propanediol, CH3CH(OH)CH2OH;
III) 1,2,3-propanetriol, (glycerol), HOCH2CH(OH)CH2OH.
A) II, III, I
B) I, II, III
C) III, I, II
D) III, II, I
E) I, III, II
II) 1,2-propanediol, CH3CH(OH)CH2OH;
III) 1,2,3-propanetriol, (glycerol), HOCH2CH(OH)CH2OH.
A) II, III, I
B) I, II, III
C) III, I, II
D) III, II, I
E) I, III, II

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
The heat of deposition equals the negative of the heat of ________.
A) fusion
B) condensation
C) sublimation
D) solidification
E) reposition
A) fusion
B) condensation
C) sublimation
D) solidification
E) reposition

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
Given the data below, determine the molar enthalpy change of vaporization of liquid Rb. Rb, P1 = 1.00 mmHg, t1 = 297°C, P2 = 400 mmHg, t2 = 620°C
A) 5.49 kJ/mol
B) 28.4 kJ/mol
C) 78.5 kJ/mol
D) 12.8 kJ/mol
E) 4.62 kJ/mol
A) 5.49 kJ/mol
B) 28.4 kJ/mol
C) 78.5 kJ/mol
D) 12.8 kJ/mol
E) 4.62 kJ/mol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
The normal boiling point of acetone is 56.2°C and the molar heat of vaporization is 32.0 kJ/mol. At what temperature will acetone boil under a pressure of 50.0 mmHg?
A) 156 °C
B) 6.0 °C
C) -6.0 °C
D) 40.7 °C
E) 73.6 °C
A) 156 °C
B) 6.0 °C
C) -6.0 °C
D) 40.7 °C
E) 73.6 °C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
How much heat would be released by the condensation of 5.40 g of steam at 100°C and the subsequent cooling of the water to 25°C? [ΔHvap = 40.7 kJ/mol at 100°C; Cp for H2O(l) is 4.18 J g-1 °C-1]
A) 12.2 kJ
B) 12.8 kJ
C) 13.9 kJ
D) 18.3 kJ
E) 23.7 kJ
A) 12.2 kJ
B) 12.8 kJ
C) 13.9 kJ
D) 18.3 kJ
E) 23.7 kJ

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
A liquid has a molar heat of vaporization of 22.7 kJ/mol. Its normal boiling point is 459K. What is the vapor pressure, in mmHg, at 70°C?
A) 102 mmHg
B) 7.48 mmHg
C) 56.8 mmHg
D) 742 mmHg
E) 580 mmHg
A) 102 mmHg
B) 7.48 mmHg
C) 56.8 mmHg
D) 742 mmHg
E) 580 mmHg

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
The factor that has the largest effect on vapor pressure is ________.
A) liquid surface area
B) molecular dipole moment
C) presence of H bonding
D) liquid mole weight
E) volume available for vapor
A) liquid surface area
B) molecular dipole moment
C) presence of H bonding
D) liquid mole weight
E) volume available for vapor

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
What would be the appropriate equilibrium temperature of a system obtained by adding 25.0 g of ice at 0°C to 250.0 mL of "hot" (80°C) coffee, assuming that the heat capacity and density of the coffee are the same as for pure water (4.18 J g-1 °C-1 and 0.997 g mL-1), and also assuming negligible heat transfer with the surroundings? [For water, ΔHfusion = 6.02 kJ/mol.]
A) 33°C
B) 40°C
C) 65°C
D) 73°C
E) 79°C
A) 33°C
B) 40°C
C) 65°C
D) 73°C
E) 79°C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
Liquid and vapor phases of a substance become indistinguishable at the ________.
A) triple point
B) normal point
C) permanent point
D) critical point
E) absolute point
A) triple point
B) normal point
C) permanent point
D) critical point
E) absolute point

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
The maximum temperature at which a gas can be liquefied is just below the ________.
A) boiling temperature
B) critical temperature
C) melting temperature
D) normal temperature
E) superheating temperature
A) boiling temperature
B) critical temperature
C) melting temperature
D) normal temperature
E) superheating temperature

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
Given the data below, determine the normal boiling point of COCl2. P1 = 100 mmHg, t1 = -35.6°C, ΔHvap = 27.4 kJ/mol
A) 278°C
B) -65.1°C
C) -36.4°C
D) 5.0°C
E) -5.0°C
A) 278°C
B) -65.1°C
C) -36.4°C
D) 5.0°C
E) -5.0°C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
Arrange the following compounds in order of increasing viscosity: I) 1-propanol, CH3CH2CH2OH;
II) 1,2-propanediol, CH3CH(OH)CH2OH;
III) 1,2,3-propanetriol, (glycerol), HOCH2CH(OH)CH2OH.
A) I, II, III
B) II, III, I
C) III, I, II
D) III, II, I
E) I, III, II
II) 1,2-propanediol, CH3CH(OH)CH2OH;
III) 1,2,3-propanetriol, (glycerol), HOCH2CH(OH)CH2OH.
A) I, II, III
B) II, III, I
C) III, I, II
D) III, II, I
E) I, III, II

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
Given the data below, determine the normal boiling point of liquid Rb. P1 = 400 mmHg, t1 = 620°C, ΔHvap = 78.5 kJ/mol
A) 374°C
B) 920°C
C) 647°C
D) 951°C
E) 678°C
A) 374°C
B) 920°C
C) 647°C
D) 951°C
E) 678°C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
The relationship between the vapor pressure of a liquid and temperature can be expressed by the Clausius-Clapeyron equation: Log[P2/P1] = [ΔHvap/R] × [1/T1 - 1/T2]
Ethanol (C2H5OH) has a normal boiling point of 78.3°C and, with ΔHvap = 39.3 kJ/mol. What is the vapor pressure of ethanol at 50.0°C?
A) 118 torr
B) 234 torr
C) 354 torr
D) 485 torr
E) 670 torr
Ethanol (C2H5OH) has a normal boiling point of 78.3°C and, with ΔHvap = 39.3 kJ/mol. What is the vapor pressure of ethanol at 50.0°C?
A) 118 torr
B) 234 torr
C) 354 torr
D) 485 torr
E) 670 torr

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
A substance has a heat of fusion of 61.5 kJ/mol and a heat of deposition of -167.4 kJ/mol. What is the heat of sublimation in kJ/mol?
A) -61.5 kJ/mol
B) 61.5 - 167.4 kJ/mol
C) 61.5 + 167.4 kJ/mol
D) 167.4 - 61.5 kJ/mol
E) 167.4 kJ/mol
A) -61.5 kJ/mol
B) 61.5 - 167.4 kJ/mol
C) 61.5 + 167.4 kJ/mol
D) 167.4 - 61.5 kJ/mol
E) 167.4 kJ/mol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
When the vapor pressure of a liquid equals atmospheric pressure, the temperature of the liquid equals ________.
A) 100°C
B) the boiling point
C) the normal boiling point
D) the vaporization point
E) the sublimation point
A) 100°C
B) the boiling point
C) the normal boiling point
D) the vaporization point
E) the sublimation point

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
The temperature at which the vapor pressure of a liquid equals the external pressure is called the ________.
A) boiling point
B) critical point
C) melting point
D) sublimation point
E) thermal point
A) boiling point
B) critical point
C) melting point
D) sublimation point
E) thermal point

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
Given the data below, determine the molar enthalpy change of vaporization of COCl2. COCl2, P1 = 40 mmHg, t1 = -50.3°C, P2 = 100 mmHg, t2 = -35.6°C
A) 0.518 kJ/mol
B) 27.4 kJ/mol
C) 4.32 kJ/mol
D) 0.928 kJ/mol
E) 0.112 kJ/mol
A) 0.518 kJ/mol
B) 27.4 kJ/mol
C) 4.32 kJ/mol
D) 0.928 kJ/mol
E) 0.112 kJ/mol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck


