Exam 12: Intermolecular Forces: Liquids and Solids
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Given the following information, calculate ΔH°(in kcal mol-1) for: Cl(g) + e- → Cl-(g) Process ΔH° (kcal mol-1)
Li(s) → Li(g) +37
Li(g) → Li+(g) + e- +124
Cl2(g) → 2Cl(g) +58 (per mole Cl2)
LiCl(s) → Li+(g) + Cl-(g) +216
2Li(s) + Cl2(g) → 2LiCl(s) -109 (per mole LiCl)
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
The property of a liquid that measures its resistance to flow is called resistivity.
Free
(True/False)
4.8/5  (32)
(32)
Correct Answer:
False
According to the phase diagram given, which of the following statements is INCORRECT? 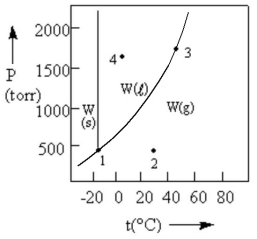
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Which of the substances below would produce the hardest, most brittle crystals?
(Multiple Choice)
5.0/5  (30)
(30)
Arrange the following compounds in order of increasing boiling point: HCl, HBr, HI.
(Multiple Choice)
4.7/5  (35)
(35)
According to the phase diagram given, which of the following statements is INCORRECT? 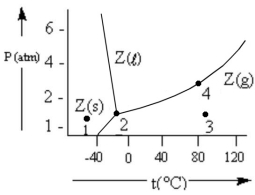
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following ionic compounds should have the highest melting point?
(Multiple Choice)
4.8/5  (40)
(40)
What is the maximum number of glycerol molecules [HOCH2CH(OH)CH2OH] that could form hydrogen bonds to a single molecule of the compound?
(Multiple Choice)
4.7/5  (40)
(40)
The temperature at which the vapor pressure of a liquid equals the external pressure is called the ________.
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following compounds is the most viscous?
CH3CH2OH, CH3CH2CH2CH3, HOCH2CH2OH, CH3OCH2CH3
(Multiple Choice)
4.8/5  (33)
(33)
Given the following information, calculate ΔH°(in kcal mol-1) for:
NaI(s) → Na+(g) + I-(g)
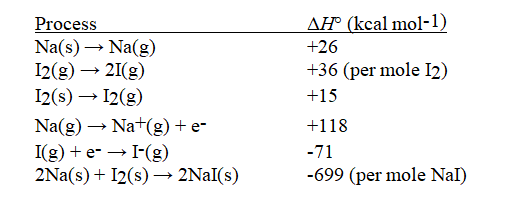
(Multiple Choice)
4.8/5  (34)
(34)
When the vapor pressure of a liquid equals atmospheric pressure, the temperature of the liquid equals ________.
(Multiple Choice)
4.9/5  (33)
(33)
Given the data below, determine the normal boiling point of COCl2. P1 = 100 mmHg, t1 = -35.6°C, ΔHvap = 27.4 kJ/mol
(Multiple Choice)
4.7/5  (38)
(38)
Which probably has the lowest boiling point at 1.0 atm pressure?
(Multiple Choice)
4.8/5  (36)
(36)
Under which of the following conditions will vaporization best occur?
(Multiple Choice)
4.8/5  (45)
(45)
Given the following information, calculate ΔH°(in kcal mol-1) for: LiCl(s) → Cl-(g) + Li+(g) Process ΔH° (kcal mol-1)
Li(s) → Li(g) +37
Li(g) → Li+(g) + e- +124
Cl2(g) → 2Cl(g) +29 (per mole Cl)
Cl(g) + e- → Cl-(g) -83
2Li(s) + Cl2(g) → 2LiCl(s) -109 (per mole LiCl)
(Multiple Choice)
4.9/5  (35)
(35)
Below are given the Lewis structures of five molecules. Which one displays the LEAST hydrogen bonding?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)