Deck 16: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 16: Aromatic Compounds
1
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 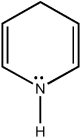

nonaromatic
2
Cyclic hydrocarbons which can be represented as structures containing alternating single and double bonds are called ________.
annulenes
3
In the molecular orbital representation of benzene, how many π molecular orbitals are present?
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8
6
4
Show how the participating p orbitals interact to form the highest energy π molecular orbital of benzene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
Show how the p orbitals overlap to generate the p4* of cyclobutadiene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 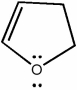


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
Classify the following compound as aromatic, antiaromatic, or nonaromatic. 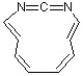


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
Provide a diagram which depicts the relative energies of the π molecular orbitals of benzene. Show which molecular orbitals are filled in benzene's ground state.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
Describe the occupied p molecular orbitals in the ground state of cyclobutadiene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
Why does benzene undergo a substitution reaction with Br2 while cyclohexene undergoes an addition reaction.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following undergoes SN2 reaction with sodium methoxide most rapidly?
A) PhCH2Br
B) Ph3CBr
C) PhCH2CH2Br
D) PhBr
E) PhCH2CH2CH2Br
A) PhCH2Br
B) Ph3CBr
C) PhCH2CH2Br
D) PhBr
E) PhCH2CH2CH2Br

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an description of benzene?
A) The CCC bond angles are all equal to 120°.
B) The molecule is planar.
C) The molecule is a 6-membered ring which contains alternating single and double carbon-carbon bonds.
D) The molecule is aromatic.
E) The molecule can be drawn as a resonance hybrid of two Kekule structures.
A) The CCC bond angles are all equal to 120°.
B) The molecule is planar.
C) The molecule is a 6-membered ring which contains alternating single and double carbon-carbon bonds.
D) The molecule is aromatic.
E) The molecule can be drawn as a resonance hybrid of two Kekule structures.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
How many distinct nodal planes which are perpendicular to the molecular plane are present in the π4* orbital of benzene?
A) 0
B) 1
C) 2
D) 4
E) 5
A) 0
B) 1
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
How many pairs of degenerate π molecular orbitals are found in benzene?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
When cyclohexene is treated with KMnO4, H2O, the syn-1,2-diol is produced. What reaction occurs when benzene is similarly treated?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
What is the bond order of the carbon-carbon bonds in benzene?
A) 0.5
B) 1
C) 1.5
D) 2
E) 3
A) 0.5
B) 1
C) 1.5
D) 2
E) 3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
What is suggested by the fact that benzene's molar heat of hydrogenation is 36 kcal less than three times the molar heat of hydrogenation of cyclohexene?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
What is the major difference between an antiaromatic and aromatic compound?
A) The structure must be cyclic for aromatic but not antiaromatic compounds?
B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring
C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat
D) Aromatic compounds cannot have a charged atom in the structure
E) Only aromatic compounds follow Huckle's rule.
A) The structure must be cyclic for aromatic but not antiaromatic compounds?
B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring
C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat
D) Aromatic compounds cannot have a charged atom in the structure
E) Only aromatic compounds follow Huckle's rule.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
Classify the following compound as aromatic, antiaromatic, or nonaromatic. 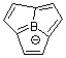


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following undergoes solvolysis in methanol most rapidly?
A) PhCH2Br
B) Ph3CBr
C) PhCH2CH2Br
D) PhBr
E) PhCH2CH2CH2Br
A) PhCH2Br
B) Ph3CBr
C) PhCH2CH2Br
D) PhBr
E) PhCH2CH2CH2Br

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following structures is aromatic?
A)
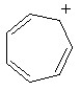
B)
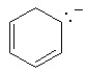
C)
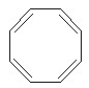
D)
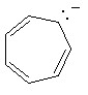
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
Provide the major resonance structures of the ion which results when the most acidic hydrogen of cyclopentadiene is lost.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
Aromatic molecules contain ________ π electrons.
A) no
B) 4n + 1 (with n an integer)
C) 4n + 2 (with n an integer)
D) 4n (with n an integer)
E) unpaired
A) no
B) 4n + 1 (with n an integer)
C) 4n + 2 (with n an integer)
D) 4n (with n an integer)
E) unpaired

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
Is the [10]annulene shown below aromatic? Explain. ![Is the [10]annulene shown below aromatic? Explain.](https://storage.examlex.com/TB6198/11eab45f_ce1c_1143_acdb_c1330321558c_TB6198_00.jpg)
![Is the [10]annulene shown below aromatic? Explain.](https://storage.examlex.com/TB6198/11eab45f_ce1c_1143_acdb_c1330321558c_TB6198_00.jpg)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
List the criteria which compounds must meet in order to be considered aromatic.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
Classify cyclopentadiene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Which is more stable, cyclobutadiene or 1,3-butadiene? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Is the all-cis form of [10]annulene aromatic? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Is cyclooctatetraene a planar molecule? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
Classify 1,3,5-heptatriene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the same as the tropylium ion?
A) cycloheptatrienyl cation
B) cycloheptatrienyl anion
C) cyclopentadienyl cation
D) cyclopentadienyl anion
E) cyclopropenyl anion
A) cycloheptatrienyl cation
B) cycloheptatrienyl anion
C) cyclopentadienyl cation
D) cyclopentadienyl anion
E) cyclopropenyl anion

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
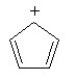
Classify the following compound as being aromatic, nonaromatic or antiaromatic. 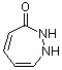


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Provide the structure of tropylium bromide.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Provide the structure of sodium cyclopentadienide.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 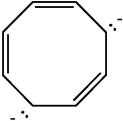


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Classify the compound below as aromatic antiaromatic, or nonaromatic. Assume planarity of the π network. 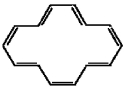


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Explain the relative acidities of cyclohexene (pKa of 46) and cyclopentadiene (pKa of 16).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following structures, if flat, would be classified as antiaromatic?
A)
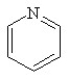
B)
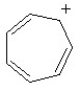
C)
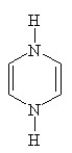
D)
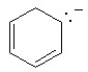
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is another name for cyclobutadiene?
A) [2]annulene
B) [4]annulene
C) [6]annulene
D) Dewar benzene
E) antibenzene
A) [2]annulene
B) [4]annulene
C) [6]annulene
D) Dewar benzene
E) antibenzene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
Classify cycloheptatriene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
Classify cycloheptatrienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
________ is similar to furan, with a sulfur atom in place of the oxygen.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Classify pyrrole as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
3-Chlorocyclopropene is solvolyzed in methanol at a much higher rate than is chlorocyclopropane. Offer an explanation.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Which sequence correctly ranks the following substrates in order of increasing reactivity in an SN1 reaction? 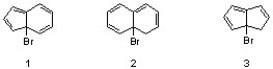
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
Rank the following in order of increasing pKa (from lowest to highest pKa) 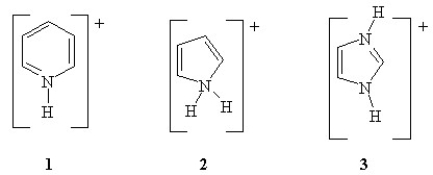
A) 1 < 2 < 3
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 3 < 1 < 2
E) 2 < 3 < 1

A) 1 < 2 < 3
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 3 < 1 < 2
E) 2 < 3 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
Is the molecule below aromatic, antiaromatic, or nonaromatic? 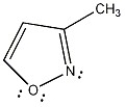


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
Why would the reaction below proceed at an extremely slow rate if at all? 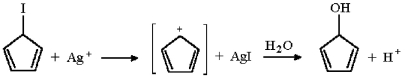


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
When cycloheptatriene is deprotonated, an anion with seven resonance forms of equal energy can be drawn. Given this fact, explain why cycloheptatriene is only slightly more acidic than propene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
Add six pi bonds to the structure below to produce the most stable isomer. 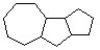


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
Which sequence ranks the indicated protons in order of increasing acidity? 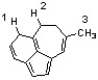
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the labeled H atoms (1 -5) in the following molecule would be predicted to be the most acidic? 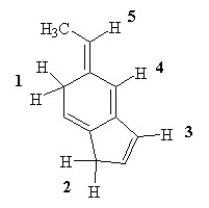
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Nitrogen's lone pair electrons occupy what type of orbital in pyridine?
A) s
B) sp
C) sp2
D) sp3
E) p
A) s
B) sp
C) sp2
D) sp3
E) p

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Classify cyclopropenyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Classify cyclopentadienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 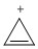


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 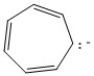


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
Which sequence ranks the indicated protons in order of increasing acidity? 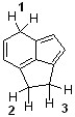
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
What compound results when cyclooctatetraene is treated with excess potassium metal?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
Classify pyridine as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
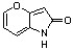
Circle and name the aromatic heterocycles in the following structure, which has been found to be effective against inflammatory diseases including Alzheimer's disease and rheumatoid arthritis (J. Med. Chem. 4728). 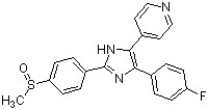


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 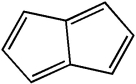


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
Is the molecule below aromatic, antiaromatic, or nonaromatic? 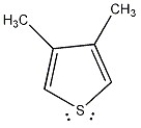


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
Name two of the three common allotropes of carbon.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds may correctly be classified as being aromatic?
(More than one answer is possible.)
A)
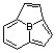
B)
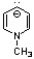
C)
D)
(More than one answer is possible.)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
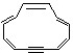
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
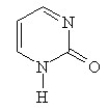
Provide the structure of pyrimidine.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Is the molecule below aromatic, antiaromatic, or nonaromatic? 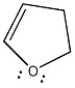


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Which sequence correctly ranks the following dienes in order of increasing reactivity in the D-A reaction? 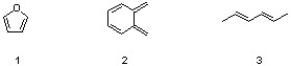
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Is the molecule below aromatic, antiaromatic, or nonaromatic? 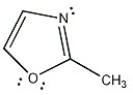


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
Is the molecule below aromatic, antiaromatic, or nonaromatic? 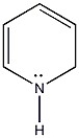


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
Classify naphthalene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 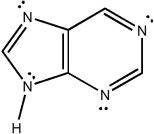


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is a fused-ring heterocycle?
A) purine
B) pyrimidine
C) benzofuran
D) indole
E) quinoline
A) purine
B) pyrimidine
C) benzofuran
D) indole
E) quinoline

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Why are researchers interested in the properties of large polynuclear aromatic hydrocarbons?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
Provide the structure of anthracene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the structure of the major organic product which results when phenanthrene is treated with Br2 in carbon tetrachloride.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
Which is more basic, pyridine or pyrrole? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck


