Exam 16: Aromatic Compounds
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
List the criteria which compounds must meet in order to be considered aromatic.
Free
(Essay)
4.8/5  (43)
(43)
Correct Answer:
1. The structure must be cyclic.
2. Each atom in the ring must have an unhybridized p orbital.
3. The structure must be planar or nearly planar so that overlap of these p orbitals is effective.
4. The π network must contain 4n + 2 electrons (where n is a whole number), so that delocalization of the π electrons results in a lowering of the molecule's electronic energy.
What is the bond order of the carbon-carbon bonds in benzene?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
C
Explain the relative acidities of cyclohexene (pKa of 46) and cyclopentadiene (pKa of 16).
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
The conjugate base of cyclopentadiene is aromatic.
What compound results when cyclooctatetraene is treated with excess potassium metal?
(Short Answer)
4.9/5  (35)
(35)
Classify cyclopropenyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network
(Short Answer)
4.8/5  (45)
(45)
Rank the following in order of increasing pKa (from lowest to highest pKa) 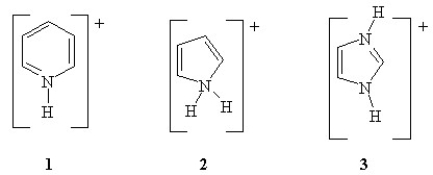
(Multiple Choice)
4.8/5  (30)
(30)
Classify pyridine as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.
(Short Answer)
4.9/5  (42)
(42)
Which of the following structures, if flat, would be classified as antiaromatic?
(Multiple Choice)
5.0/5  (27)
(27)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 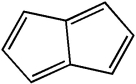
(Short Answer)
4.9/5  (39)
(39)
When cycloheptatriene is deprotonated, an anion with seven resonance forms of equal energy can be drawn. Given this fact, explain why cycloheptatriene is only slightly more acidic than propene.
(Essay)
4.9/5  (34)
(34)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 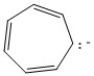
(Short Answer)
4.7/5  (39)
(39)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 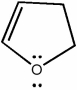
(Short Answer)
4.9/5  (39)
(39)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)