Deck 16: Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 16: Aromatic Compounds
1
Cyclic hydrocarbons which can be represented as structures containing alternating single and double bonds are called ________.
annulenes
2
Even assuming the molecule below is planar, it would still be classified as non-aromatic. Why? 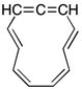

The center carbon of the allene is sp hybridized and the p orbitals are 90° to each other. There is no overlap of the pi system.
3
When toluene is treated with bromine (shown below), the bromine doesn't react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes place. Why? 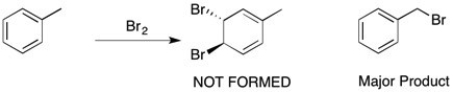

Addition of Br2 to the double bonds of benzene is highly unfavorable because it would result in a nonaromatic product (aromatic stabilization would be lost). Instead, a radical reaction to form a highly stabilized radical would occur.
4
How many pairs of degenerate π molecular orbitals are found in benzene?
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
The bond lengths in a substituted cyclobutadiene compounds were determined by computational methods to display very unequal bond lengths (1.354 and 1.608
A). What does this suggest about resonance structures in this compound? (J. Phys. Chem. A, 2000, 104, 1246-1255)
A). What does this suggest about resonance structures in this compound? (J. Phys. Chem. A, 2000, 104, 1246-1255)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an incorrect description of benzene?
A) The CCC bond angles are all equal to 120°.
B) The molecule is planar.
C) The molecule is a 6-membered ring which contains alternating single and double carbon-carbon bonds.
D) The molecule is aromatic.
E) The molecule can be drawn as a resonance hybrid of two Kekule structures.
A) The CCC bond angles are all equal to 120°.
B) The molecule is planar.
C) The molecule is a 6-membered ring which contains alternating single and double carbon-carbon bonds.
D) The molecule is aromatic.
E) The molecule can be drawn as a resonance hybrid of two Kekule structures.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
How many distinct nodal planes which are perpendicular to the molecular plane are present in the π4* orbital of benzene?
A) 0
B) 1
C) 2
D) 4
E) 5
A) 0
B) 1
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
Show how the participating p orbitals interact to form the highest energy π molecular orbital of benzene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major difference between an antiaromatic and aromatic compound?
A) The structure must be cyclic for aromatic but not antiaromatic compounds.
B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring.
C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat.
D) Aromatic compounds cannot have a charged atom in the structure.
E) Only aromatic compounds follow Huckle's rule.
A) The structure must be cyclic for aromatic but not antiaromatic compounds.
B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring.
C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat.
D) Aromatic compounds cannot have a charged atom in the structure.
E) Only aromatic compounds follow Huckle's rule.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
Classify the following compound as aromatic, antiaromatic, or nonaromatic. 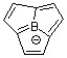


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
Which would have a smaller heat of hydrogenation? Circle your answer. 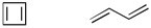
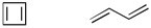

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
Use the polygon rule to draw the MO energy diagram of the cyclononatetraenyl anion. Assuming planarity, would this ion be aromatic or antiaromatic?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
What is the bond order of the carbon-carbon bonds in benzene?
A) 0.5
B) 1
C) 1.5
D) 2
E) 3
A) 0.5
B) 1
C) 1.5
D) 2
E) 3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
In the molecular orbital representation of benzene, how many π molecular orbitals are present?
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
When cyclohexene is treated with KMnO4, H2O, the syn-1,2-diol is produced. What reaction occurs when benzene is similarly treated?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
The MO energy diagram for cyclobutadiene is shown below. Fill in the π electrons for the neutral compound. Does cyclobutadiene react like a cation, anion or radical in reactions? 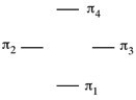


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
What is suggested by the fact that benzene's molar heat of hydrogenation is 36 kcal less than three times the molar heat of hydrogenation of cyclohexene?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
Show how the p orbitals overlap to generate the π4* of cyclobutadiene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
Provide a diagram which depicts the relative energies of the π molecular orbitals of benzene. Show which molecular orbitals are filled in benzene's ground state.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
Describe the occupied π molecular orbitals in the ground state of cyclobutadiene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is another name for cyclobutadiene?
A) [2]annulene
B) [4]annulene
C) [6]annulene
D) Dewar benzene
E) antibenzene
A) [2]annulene
B) [4]annulene
C) [6]annulene
D) Dewar benzene
E) antibenzene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
List the criteria which compounds must meet in order to be considered aromatic.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
Classify cyclopentadiene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
Provide the structure of sodium cyclopentadienide.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
Explain the relative acidities of cyclohexene (pKa of 46) and cyclopentadiene (pKa of 16).

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the same as the tropylium ion?
A) cycloheptatrienyl cation
B) cycloheptatrienyl anion
C) cyclopentadienyl cation
D) cyclopentadienyl anion
E) cyclopropenyl anion
A) cycloheptatrienyl cation
B) cycloheptatrienyl anion
C) cyclopentadienyl cation
D) cyclopentadienyl anion
E) cyclopropenyl anion

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Is cyclooctatetraene a planar molecule? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Classify 1,3,5-heptatriene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Classify cycloheptatriene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
Is the [10]annulene shown below aromatic? Explain. ![Is the [10]annulene shown below aromatic? Explain.](https://storage.examlex.com/TB6199/11eab5e1_c97f_8d3a_9bae_df56a1e269c3_TB6199_00.jpg)
![Is the [10]annulene shown below aromatic? Explain.](https://storage.examlex.com/TB6199/11eab5e1_c97f_8d3a_9bae_df56a1e269c3_TB6199_00.jpg)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
Aromatic molecules contain ________ π electrons.
A) no
B) 4n + 1 (with n an integer)
C) 4n + 2 (with n an integer)
D) 4n (with n an integer)
E) unpaired
A) no
B) 4n + 1 (with n an integer)
C) 4n + 2 (with n an integer)
D) 4n (with n an integer)
E) unpaired

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 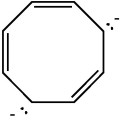


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following structures is aromatic?
A)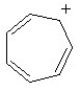
B)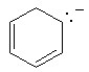
C)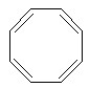
D)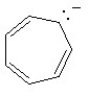
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Classify the compound below as aromatic antiaromatic, or nonaromatic. Assume planarity of the π network. 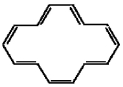


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Which is more stable, cyclobutadiene or 1,3-butadiene? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Classify the following compound as being aromatic, nonaromatic or antiaromatic. 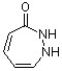


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Is the all-cis form of [10]annulene aromatic? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the structure of tropylium bromide.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following structures, if flat, would be classified as antiaromatic?
A)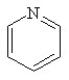
B)
C)
D)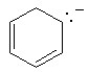
E)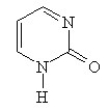
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the major resonance structures of the ion which results when the most acidic hydrogen of cyclopentadiene is lost.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
________ is similar to furan, with a sulfur atom in place of the oxygen.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Classify cyclopentadienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
Classify cyclopropenyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Which sequence ranks the indicated protons in order of increasing acidity? 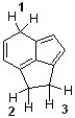
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
When cycloheptatriene is deprotonated, an anion with seven resonance forms of equal energy can be drawn. Given this fact, explain why cycloheptatriene is only slightly more acidic than propene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
Classify cycloheptatrienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the labeled H atoms (1 -5) in the following molecule would be predicted to be the most acidic? 
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
Nitrogen's lone pair electrons occupy what type of orbital in pyridine?
A) s
B) sp
C) sp2
D) sp3
E) p
A) s
B) sp
C) sp2
D) sp3
E) p

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
Rank the following in order of increasing pKa (from lowest to highest pKa). 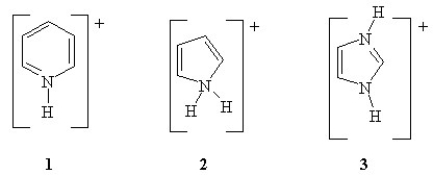
A) 1 < 2 < 3
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 3 < 1 < 2
E) 2 < 3 < 1

A) 1 < 2 < 3
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 3 < 1 < 2
E) 2 < 3 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
Use resonance structures to explain why the compound shown below is considered to be antiaromatic. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
Classify pyrrole as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Is the molecule below aromatic, antiaromatic, or nonaromatic? 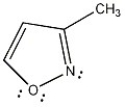


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Which sequence correctly ranks the following substrates in order of increasing reactivity in an SN1 reaction? 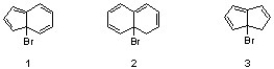
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Styrene (shown below) has 8 π electrons but is aromatic. Why? 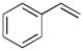


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 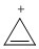


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Why would the reaction below proceed at an extremely slow rate if at all? 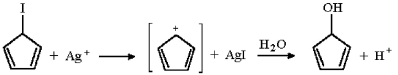


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
3-Chlorocyclopropene is solvolyzed in methanol at a much higher rate than is chlorocyclopropane. Offer an explanation.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
Which sequence ranks the indicated protons in order of increasing acidity? 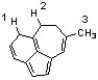
A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

A) 1 < 2 < 3
B) 2 < 3 < 1
C) 3 < 1 < 2
D) 3 < 2 < 1

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 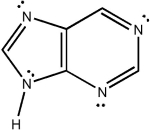


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
Which is more basic, pyridine or pyrrole? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following compounds may correctly be classified as being aromatic?
(More than one answer is possible.)
A)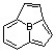
B)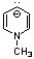
C)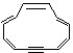
D)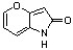
(More than one answer is possible.)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
Is the molecule below aromatic, antiaromatic, or nonaromatic? 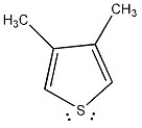


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is not a fused-ring heterocycle?
A) purine
B) pyrimidine
C) benzofuran
D) indole
E) quinoline
A) purine
B) pyrimidine
C) benzofuran
D) indole
E) quinoline

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
Is the molecule below aromatic, antiaromatic, or nonaromatic? 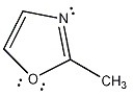


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Circle and name the aromatic heterocycles in the following structure, which has been found to be effective against inflammatory diseases including Alzheimer's disease and rheumatoid arthritis (J. Med. Chem. 2007, 4728). 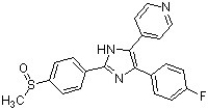


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
Classify naphthalene as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the structure of anthracene.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 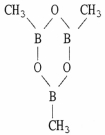


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Pyrene has been tentatively identified in the interstellar medium. Use Huckel Rule to determine is pyrene is aromatic or antiaromatic (assuming planarity of the π system). 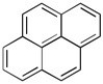


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Which orbital is the lone pair of each N in, sp2 or p? 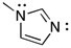


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 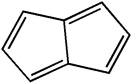


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
Name two of the three common allotropes of carbon.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
Provide the structure of pyrimidine.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Is the molecule below aromatic, antiaromatic, or nonaromatic? 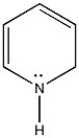


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Is the molecule below aromatic, antiaromatic, or nonaromatic? 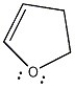


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
Provide the structure of the major organic product which results when phenanthrene is treated with Br2 in carbon tetrachloride.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Classify pyridine as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 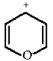


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck


