Exam 16: Aromatic Compounds
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Is cyclooctatetraene a planar molecule? Explain.
Free
(Essay)
4.9/5  (33)
(33)
Correct Answer:
No. In addition to increasing other forms of strain, a planar conformation would make this molecule antiaromatic.
Provide the name of the compound shown below. 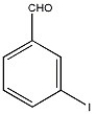
Free
(Short Answer)
4.7/5  (23)
(23)
Correct Answer:
m-iodobenzaldehyde or 3-iodobenzaldehyde
Show how the participating p orbitals interact to form the highest energy π molecular orbital of benzene.
Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
All adjacent interactions are antibonding. 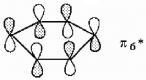
Which of the following structures, if flat, would be classified as antiaromatic?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following compounds may correctly be classified as being aromatic?
(More than one answer is possible.)
(Multiple Choice)
4.8/5  (32)
(32)
Classify the compound below as aromatic antiaromatic, or nonaromatic. Assume planarity of the π network. 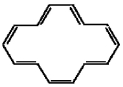
(Short Answer)
4.7/5  (39)
(39)
Classify cyclopropenyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network
(Short Answer)
4.7/5  (34)
(34)
When toluene is treated with bromine (shown below), the bromine doesn't react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes place. Why? 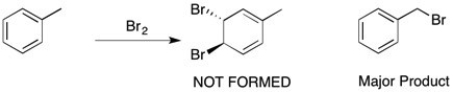
(Essay)
4.8/5  (30)
(30)
When cyclohexene is treated with KMnO4, H2O, the syn-1,2-diol is produced. What reaction occurs when benzene is similarly treated?
(Short Answer)
4.9/5  (34)
(34)
Circle and name the aromatic heterocycles in the following structure, which has been found to be effective against inflammatory diseases including Alzheimer's disease and rheumatoid arthritis (J. Med. Chem. 2007, 4728). 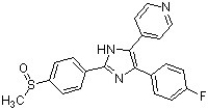
(Essay)
4.7/5  (37)
(37)
Describe the occupied π molecular orbitals in the ground state of cyclobutadiene.
(Essay)
4.8/5  (34)
(34)
An unknown compound gives the following spectral data. Propose a structure that is consistent with this information. In addition, show the fragmentations that give the 91 and 122 peaks in the MS.
MS (m/z): 91, 108 and 122 (M+)
1H NMR (δ): 7.05(2H, d), 6.78 (2H, d), 3.7 (3H, s), 2.26 (3H, s)
(Essay)
4.9/5  (32)
(32)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)